Bioresorbable, wireless dual stimulator for peripheral nerve regeneration
- PMID: 40404648
- PMCID: PMC12098704
- DOI: 10.1038/s41467-025-59835-7
Bioresorbable, wireless dual stimulator for peripheral nerve regeneration
Abstract
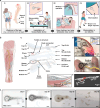
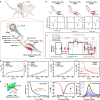
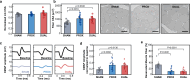
Wireless bioresorbable electrical stimulators have broad potential as therapeutic implants. Such devices operate for a clinically relevant duration and then harmlessly dissolve, eliminating the need for surgical removal. A representative application is in treating peripheral nerve injuries through targeted stimulation at either proximal or distal sites, with operation for up to one week. This report introduces enhanced devices with additional capabilities: (1) simultaneous stimulation of both proximal and distal sites, and (2) robust operation for as long as several months, all achieved with materials that naturally resorb by hydrolysis in surrounding biofluids. Systematic investigations of the materials and design aspects highlight the key features that enable dual stimulation and with enhanced stability. Animal model studies illustrate beneficial effects in promoting peripheral nerve regeneration, as quantified by increased total muscle and muscle fiber cross-sectional area and compound muscle action potentials. These findings expand the clinical applications of bioresorbable stimulators, particularly for long-term nerve regeneration and continuous neuromodulation-based monitoring.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


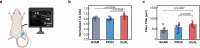
References
-
- Hardy, P. B., Wang, B. Y., Chan, K. M., Webber, C. A. & Senger, J. L. B. The use of electrical stimulation to enhance recovery following peripheral nerve injury. Muscle Nerve70, 1151–1162 (2024). - PubMed
-
- Gordon, T., Amirjani, N., Edwards, D. C. & Chan, K. M. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp. Neurol.223, 192–202 (2010). - PubMed
-
- Wong, J. N., Olson, J. L., Morhart, M. J. & Chan, K. M. Electrical stimulation enhances sensory recovery: a randomized controlled trial. Ann. Neurol.77, 996–1006 (2015). - PubMed
-
- Power, H. A., Morhart, M. J., Olson, J. L. & Chan, K. M. Postsurgical electrical stimulation enhances recovery following surgery for severe cubital tunnel syndrome: a double-blind randomized controlled trial. Neurosurgery86, 769–777 (2020). - PubMed
MeSH terms
Grants and funding
- R01NS126918/U.S. Department of Health & Human Services | NIH | Center for Information Technology (Center for Information Technology, National Institutes of Health)
- R01NS126918/U.S. Department of Health & Human Services | NIH | Center for Information Technology (Center for Information Technology, National Institutes of Health)
- HI19C1348/Korea Health Industry Development Institute (KHIDI)
- Haythornthwaite Foundation Research Initiation Grant/American Society of Mechanical Engineers (ASME)
- R03HD101090/U.S. Department of Health & Human Services | NIH | Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)
LinkOut - more resources
Full Text Sources
Medical

