Rational selection of the monoclonal α-synuclein antibody amlenetug (Lu AF82422) for the treatment of α-synucleinopathies
- PMID: 40404755
- PMCID: PMC12098740
- DOI: 10.1038/s41531-024-00849-1
Rational selection of the monoclonal α-synuclein antibody amlenetug (Lu AF82422) for the treatment of α-synucleinopathies
Abstract
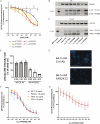
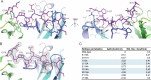
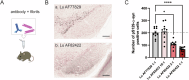
Amlenetug (Lu AF82422) is a human monoclonal antibody targeting α-synuclein in clinical development for multiple system atrophy. We describe a series of studies that characterize its functional properties and supported its selection as a viable clinical candidate. Amlenetug inhibits seeding induced in mouse primary neurons by various α-synuclein fibrillar assemblies and by aggregates isolated from MSA brain homogenate. In vivo, both co-injection of amlenetug with α-synuclein assemblies in mouse brain and peripheral administration inhibit α-synuclein seeding. Amlenetug inhibits uptake of α-synuclein seeds as well as accumulation of C-terminal truncated α-synuclein seeds and demonstrates binding to monomeric, aggregated, and truncated forms of human α-synuclein. The epitope of amlenetug was mapped to amino acids 112-117 and further characterized by crystallographic structure analysis. Based on our data, we hypothesize that targeting α-synuclein will potentially slow further disease progression by inhibiting further pathology development but be without impact on established pathology and symptoms.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: P.K., F.S., K.W., M.L., L.D., M.A., L.B., B.O.K., L.R.O., S.V., A.J., M.N.H., D.S.M.D., K.B.-A., K.F. are full-time employees of H. Lundbeck A/S. A.-L.B., I.M., S.N., T.T.E., J.B.S., K.J.A., L.Ø.P., S.C., J.E., J.N.S., P.G.W.-L. were full-time employees of H. Lundbeck A/S at the time of this work. E.N.V.D.B., R.R., D.S., P.P. were full-time employees of Genmab BV at the time of this work. The research was funded by H. Lundbeck A/S, Copenhagen, Denmark.
Figures



References
-
- Papp, M. I. & Lantos, P. L. The distribution of oligodendroglial inclusions in multiple system atrophy and its relevance to clinical symptomatology. Brain J. Neurol.117, 235–243 (1994). - PubMed
-
- Papp, M. I. & Lantos, P. L. Accumulation of tubular structures in oligodendroglial and neuronal cells as the basic alteration in multiple system atrophy. J. Neurol. Sci.107, 172–182 (1992). - PubMed
-
- Engelhardt, E. Lafora and Trétiakoff: the naming of the inclusion bodies discovered by Lewy. Arq. Neuropsiquiatr.75, 751–753 (2017). - PubMed
-
- Lewy, F. H. in Handbuch der Neurologie, Vol. 3 (ed. Lewandowsky, M.) Ch. I. Pathologische Anatomie, 920–958 (Springer, 1912).
-
- Papp, M. I., Kahn, J. E. & Lantos, P. L. Glial cytoplasmic inclusions in the CNS of patients with multiple system atrophy (striatonigral degeneration, olivopontocerebellar atrophy and Shy-Drager syndrome). J. Neurol. Sci.94, 79–100 (1989). - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

