Revitalizing Pleurotus eryngii polysaccharides: gamma irradiation boosts antidiabetic and antioxidant potential
- PMID: 40404997
- PMCID: PMC12098236
- DOI: 10.1186/s40643-025-00854-z
Revitalizing Pleurotus eryngii polysaccharides: gamma irradiation boosts antidiabetic and antioxidant potential
Abstract
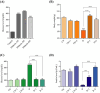
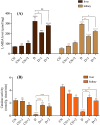
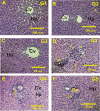
Polysaccharides derived from Pleurotus eryngii possess various bioactive properties, including antioxidant, antidiabetic, anti-inflammatory, and immunomodulatory effects. In this study, polysaccharides were extracted from P. eryngii fruiting bodies and exposed to gamma irradiation at doses of 50 and 100 kGy, with a dose rate of 5 kGy/h. The surface morphology of the polysaccharide irradiated at 100 kGy exhibited numerous pores and a smaller flake structure compared to those irradiated at 50 kGy and the non-irradiated sample. 1H and 13C NMR spectra of all samples indicated that both irradiated and non-irradiated polysaccharides exhibited α- and β-configurations, with signals corresponding to C1-C5 clearly observed. HPLC analysis of the polysaccharides revealed that glucose (75.23%), galactose (4.96%), glucuronic acid (1.38%), ribose (0.94%), rhamnose (2.35%), and mannose (3.87%) are the main components. All polysaccharides demonstrated antioxidant activity, which increased with concentration. Both non-irradiated and irradiated polysaccharides exhibited antidiabetic effects, significantly reducing blood glucose levels, and restoring insulin level with superiority of irradiated polysaccharides. Additionally, they significantly elevated body weight, slightly reduced MDA levels, and markedly enhanced catalase activity in treated rats compared to diabetic controls. The antidiabetic effects of the polysaccharides were further confirmed by histopathological examination of the pancreas and liver sections from polysaccharide-treated diabetic rats. This suggests that irradiation, by reducing the molecular weight of polysaccharides, enhances their bioavailability and efficacy in modulating glucose metabolism.
Keywords: Antioxidant; Diabetic; Gamma radiation; Histopathology; Mushroom; Polysaccharides.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Animal Ethics Committee (IAEC) of Tanta University’s Faculty of Science (IACUC-SCI-TU-0170). Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures




References
-
- Abd El-Zaher E, Tousson EM, Mostafa AA, El-Gaar M E (2022) Production of Endo polysaccharides from cultivated pleurotus eryngii fruiting bodies. Delta J Sci 44:135–144. 10.21608/djs.2022.127995.1020 - DOI
-
- Bakaç MS, Dogan A, Yılmaz MA, Altındag F, Donmez F, Battal A (2023) Ameliorative effects of Scutellaria pinnatifida subsp. Pichleri (Stapf) Rech.f. Extract in streptozotocin-induced diabetic rats: chemical composition, biochemical and histopathological evaluation. BMC Complement Med Ther 23:410. 10.1186/s12906-023-04252-w - DOI - PMC - PubMed
-
- Balaji P, Madhanraj R, Rameshkumar K, Veeramanikandan V, Eyini M, Arun A, Thulasinathan B, Al Farraj DA, Elshikh MS, Alokda AM, Mahmoud AH, Tack J-C, Kim H-J (2020) Evaluation of antidiabetic activity of pleurotus pulmonarius against streptozotocin-nicotinamide induced diabetic Wistar albino rats. Saudi J Biol Sci 27:913–924. 10.1016/j.sjbs.2020.01.027 - DOI - PMC - PubMed
-
- Dedousi M, Melanouri E-M, Diamantopoulou P (2023) Carposome productivity of pleurotus ostreatus and pleurotus eryngii growing on agro-industrial residues enriched with nitrogen, calcium salts and oils. Carbon Resour Convers 6:150–165. 10.1016/j.crcon.2023.02.001 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

