Retrospective analysis of pembrolizumab-related adverse reactions and death outcomes based on the FAERS database
- PMID: 40405105
- PMCID: PMC12096721
- DOI: 10.1186/s12885-025-14342-2
Retrospective analysis of pembrolizumab-related adverse reactions and death outcomes based on the FAERS database
Abstract
Objective: This study aimed to analyze the characteristics of adverse reactions in cancer patients treated with Pembrolizumab based on the U.S. Food and Drug Administration's Adverse Event Reporting System (FAERS) database, and to assess the characteristics and risk factors of fatality reports.
Methods: The study data was sourced from the FAERS database, collecting adverse event reports related to Pembrolizumab from 2013 to June 2024. The main analysis variables included gender, age, cancer type, country, reporter type, and adverse reaction outcomes. Descriptive statistics, univariate analysis, and multivariate Logistic regression models were used to assess the relationship between each variable and fatal outcome.
Results: A total of 46,883 adverse reactions were collected, including 5,483 reports with fatal outcomes. The number of events has been increasing since 2013, especially peaking in 2022 and 2023. The United States and Japan had the highest number of adverse reaction reports. The number of serious events reported increased significantly with age, especially in the 51-65 and 66-80 age groups. The age of patients who died was concentrated in the elderly group (≥ 65 years old), and the median treatment duration time of pembrolizumab was 17 days. Analysis showed that gender (OR = 0.75; 95%CI: 0.71-0.80, p < 0.01), age (OR = 0.89; 95%CI: 0.84-0.96, p < 0.01), and ingredients count (OR = 1.92; 95%CI: 1.84-2.01, p < 0.01) were significantly associated with the treatment duration of pembrolizumab.
Conclusion: The serious adverse reactions in cancer patients treated with Pembrolizumab are closely related to patient individual characteristics and cancer types. It is necessary to strengthen the monitoring of high-risk groups such as the elderly in clinical treatment to reduce the risk of fatal outcomes.
Keywords: Adverse reactions; FAERS; Immunotherapy; Pembrolizumab; Prognostic factors.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. As this research was based on publicly available data, no ethical approval was required. Informed consent was not applicable as the study was retrospective design. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
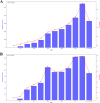
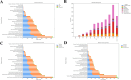

References
-
- Doi T, Piha-Paul SA, Jalal SI, Saraf S, Lunceford J, Koshiji M, Bennouna JJJCO. Safety and antitumor activity of the anti–programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. 2018, 36(1):61–7. - PubMed
-
- Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, Weber JS, Joshua AM, Hwu W-J, Gangadhar TCJTL. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. 2014, 384(9948):1109–17. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

