Patient characteristics and pharmacologic treatment patterns in antifibrotic-treated patients with fibrosing interstitial lung diseases: real-world results from a claims database
- PMID: 40405141
- PMCID: PMC12096679
- DOI: 10.1186/s12890-025-03713-x
Patient characteristics and pharmacologic treatment patterns in antifibrotic-treated patients with fibrosing interstitial lung diseases: real-world results from a claims database
Abstract
Background: Antifibrotics have been approved for use in many countries, including Japan, based on the results of several phase III clinical trials in patients with IPF, SSc-ILD, and PPF, which showed slower lung function decline with antifibrotic treatment. There is a paucity of information on the real-world use of antifibrotics in clinical practice.
Methods: Baseline characteristics, comorbidities, and drugs used prior to and concomitant with antifibrotics were collected for patients with IPF, SSc-ILD, and PPF using a health insurance claims database in Japan from 1 January 2013 to 30 June 2023. Descriptive statistics were generated for all study variables.
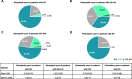
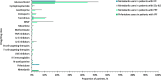
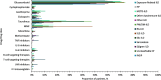
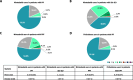
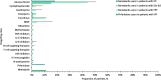
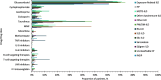
Results: This study included 657 nintedanib users with IPF; 418 pirfenidone users with IPF; 4160 nintedanib users with PPF; 18,403 users of glucocorticoids/immunosuppressants for ILD treatment with PPF; 676 nintedanib users with SSc-ILD; and 698 users of glucocorticoids/immunosuppressants for ILD treatment with SSc-ILD. At index, pirfenidone users with IPF were the oldest (mean [SD] 74.8 [7.3] years), and nintedanib users with SSc-ILD were the youngest (mean [SD] 65.6 [11.7] years). In nintedanib users with IPF, 76.7% were prescribed nintedanib as monotherapy, and 75.6% of pirfenidone users were prescribed pirfenidone, as monotherapy. In patients with IPF, 75.2% were prescribed nintedanib, and 76.1% were prescribed pirfenidone, as first-line therapy. In patients with SSc-ILD, 34.9% were prescribed nintedanib as monotherapy for ILD treatment, and 38.6% as first-line therapy. Approximately half of patients with PPF were prescribed nintedanib concomitantly with other glucocorticoids/immunosuppressant drugs, and after one or more glucocorticoids/immunosuppressant drugs. The most common concomitant drug in all patient groups was glucocorticoids. In patients with IPF, 18.6% of nintedanib users and 18.2% of pirfenidone users were prescribed glucocorticoids concomitantly. Concomitant glucocorticoid use was 52.7% for nintedanib users with SSc-ILD, and 44.1% for nintedanib users with PPF.
Conclusions: These results provide real-world evidence of antifibrotic use in clinical practice. Most patients with IPF were prescribed antifibrotics as monotherapy for ILD treatment whereas antifibrotics were used concomitantly with glucocorticoids/immunosuppressants in many patients with SSc-ILD and PPF. While most patients with IPF were prescribed antifibrotics as first-line therapy, patients with SSc-ILD and PPF were more likely to be prescribed nintedanib as second-line or later-line treatment after glucocorticoids/immunosuppressants.
Keywords: Antifibrotics; Claims data; Database; Japan; Nintedanib; Post-marketing; Progression; Pulmonary fibrosis; Real-world data.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was designed, conducted, and reported using best practice guidelines, with Institutional Review Board (IRB) approval from the Takahashi Clinic Ethics Committee on December 5, 2023 (IRB approval number: LNW00207). Hence, all objectives and methods were pre-specified and executed according to the study protocol. This study used anonymised processed information from the Medical Data Vision database and therefore informed consent to participate was deemed unnecessary according to Ethical Guidelines for Medical and Biological Research Involving Human Subjects (2023 Revision) by the Ministry of Education, Culture, Sports, Science and Technology, Ministry of Health, Labour and Welfare, and Ministry of Economy, Trade and Industry, in Japan. In addition, as this study used anonymised processed information from the Medical Data Vision database, the Declaration of Helsinki is not applicable. Consent for publication: Not applicable. Competing interests: Yasuhiro Kondoh has received consulting fees from Asahi Kasei Pharma Corp, Boehringer Ingelheim, Chugai Pharmaceutical Co., Ltd., Healios K.K., Janssen Pharmaceutical KK, Shionogi Co, Ltd., and Taiho Pharmaceutical Co., Ltd., and serves on speaker bureaus for Asahi Kasei Pharma Corp, Boehringer Ingelheim, Eisai Co, Ltd, Bristol Myers Squibb, Janssen Pharmaceutical K.K., KYORIN Pharmaceutical Co, Ltd, Mitsubishi Tanabe Pharma, NIPPON SHINYAKU CO., LTD, Novartis Pharma KK, Shionogi Co, Ltd., and Teijin Pharma Ltd. Tomohiro Ito, Hana Kimura, and Haikun Bao are employees of Boehringer Ingelheim. Masataka Kuwana has received consulting fees, speaking fees, and research grants from AbbVie, argenx, Asahi Kasei, AstraZeneca, Astellas, Boehringer Ingelheim, Chugai, Corbus, Eisai, GlaxoSmithKline, Horizon, Janssen, Kissei, MBL, Mitsubishi Tanabe, Mochida, Nippon Shinyaku, Ono Pharmaceuticals, Pfizer, and Taisho. Tomohiro Ito, Hana Kimura, and Haikun Bao are employees of Boehringer Ingelheim.
Figures
Similar articles
-
Nintedanib: A Review in Fibrotic Interstitial Lung Diseases.Drugs. 2021 Apr;81(5):575-586. doi: 10.1007/s40265-021-01487-0. Epub 2021 Mar 25. Drugs. 2021. PMID: 33765296 Free PMC article. Review.
-
Possible value of antifibrotic drugs in patients with progressive fibrosing non-IPF interstitial lung diseases.BMC Pulm Med. 2019 Nov 12;19(1):213. doi: 10.1186/s12890-019-0937-0. BMC Pulm Med. 2019. PMID: 31718637 Free PMC article.
-
Antifibrotics in rheumatoid arthritis-associated interstitial lung disease - real-world data from a nationwide cohort.ARP Rheumatol. 2024 Jul-Sep;3(3):182-188. doi: 10.63032/POPM9413. ARP Rheumatol. 2024. PMID: 39368099 English.
-
Nintedanib for the treatment of systemic sclerosis-associated interstitial lung disease.Expert Rev Clin Immunol. 2020 Jun;16(6):547-560. doi: 10.1080/1744666X.2020.1777857. Epub 2020 Jun 17. Expert Rev Clin Immunol. 2020. PMID: 32506975 Review.
-
The impact of antifibrotic use on long-term clinical outcomes in the pulmonary fibrosis foundation registry.Respir Res. 2024 Jun 21;25(1):255. doi: 10.1186/s12931-024-02883-2. Respir Res. 2024. PMID: 38907239 Free PMC article.
References
-
- Raghu G, Chen SY, Yeh WS, Maroni B, Li Q, Lee YC, Collard HR. Idiopathic pulmonary fibrosis in US Medicare beneficiaries aged 65 years and older: incidence, prevalence, and survival, 2001–11. Lancet Respir Med. 2014;2(7):566–72. - PubMed
-
- George PM, Spagnolo P, Kreuter M, Altinisik G, Bonifazi M, Martinez FJ, Molyneaux PL, Renzoni EA, Richeldi L, Tomassetti S, et al. Progressive fibrosing interstitial lung disease: clinical uncertainties, consensus recommendations, and research priorities. Lancet Respir Med. 2020;8(9):925–34. - PubMed
-
- Raghu G, Remy-Jardin M, Richeldi L, Thomson CC, Inoue Y, Johkoh T, Kreuter M, Lynch DA, Maher TM, Martinez FJ, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2022;205(9):e18–47. - PMC - PubMed
-
- Flaherty KR, Wells AU, Cottin V, Devaraj A, Walsh SLF, Inoue Y, Richeldi L, Kolb M, Tetzlaff K, Stowasser S, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381(18):1718–27. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

