N-phenylmaleimide induces bioenergetic switch and suppresses tumor growth in glioblastoma tumorspheres by inhibiting SLC25A11
- PMID: 40405188
- PMCID: PMC12096590
- DOI: 10.1186/s12935-025-03813-y
N-phenylmaleimide induces bioenergetic switch and suppresses tumor growth in glioblastoma tumorspheres by inhibiting SLC25A11
Abstract
Background: Glioblastoma (GBM) is a highly resistant tumor, and targeting its bioenergetics could be a potential treatment strategy. GBM cells depend on cytosolic nicotinamide adenine dinucleotide (NADH), which is transported into the mitochondria via the malate-aspartate shuttle (MAS) for ATP production. N-phenylmaleimide (KN612) is a MAS inhibitor that targets SLC25A11, an antiporter protein of the MAS. Therefore, this study investigated the effects of KN612 in GBM treatment using in vitro and in vivo models.
Methods: We examined the biological effects of KN612 in GBM tumorspheres (TSs), including its effects on cell viability, ATP level, cell cycle, stemness, invasive properties, energy metabolic pathways, and transcriptomes. Additionally, we investigated the in vivo efficacy of KN612 in a mouse orthotopic xenograft model.
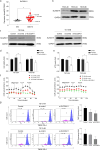
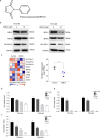
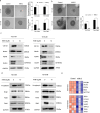
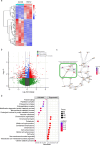
Results: Transcriptomic analysis showed that SLC25A11 mRNA expression was significantly higher in GBM TSs than in normal human astrocytes. Additionally, siRNA-mediated SLC25A11 knockdown and KN612-mediated MAS inhibition decreased the oxygen consumption rate, ATP levels, mitochondrial activity, and cell viability in GBM TSs and decreased the stemness and invasion ability of GBM cells. Moreover, gene ontology functional annotation indicated that KN612 treatment inhibited cell-cycle and mitotic processes. Furthermore, KN612 treatment reduced tumor size and prolonged survival in an orthotopic xenograft model.
Conclusions: Targeting GBM bioenergetics using KN612 may represent a novel and effective approach for GBM treatment.
Keywords: Bioenergetics; Glioblastoma; KN612; Malate-aspartate shuttle; SLC25A11.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All experiments involving animals followed the ethical standards of the institution or practice at which the studies were conducted. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Regulation of bioenergetics through dual inhibition of aldehyde dehydrogenase and mitochondrial complex I suppresses glioblastoma tumorspheres.Neuro Oncol. 2018 Jun 18;20(7):954-965. doi: 10.1093/neuonc/nox243. Neuro Oncol. 2018. PMID: 29294080 Free PMC article.
-
A novel biguanide (IM1761065) inhibits bioenergetics of glioblastoma tumorspheres.J Neurooncol. 2022 Jan;156(1):139-151. doi: 10.1007/s11060-021-03903-7. Epub 2021 Nov 22. J Neurooncol. 2022. PMID: 34811601
-
Inhibition of glioblastoma tumorspheres by combined treatment with 2-deoxyglucose and metformin.Neuro Oncol. 2017 Feb 1;19(2):197-207. doi: 10.1093/neuonc/now174. Neuro Oncol. 2017. PMID: 27571886 Free PMC article.
-
Dual inhibition of CPT1A and G6PD suppresses glioblastoma tumorspheres.J Neurooncol. 2022 Dec;160(3):677-689. doi: 10.1007/s11060-022-04189-z. Epub 2022 Nov 17. J Neurooncol. 2022. PMID: 36396930
-
Mitochondrial Substrate-Level Phosphorylation as Energy Source for Glioblastoma: Review and Hypothesis.ASN Neuro. 2018 Jan-Dec;10:1759091418818261. doi: 10.1177/1759091418818261. ASN Neuro. 2018. PMID: 30909720 Free PMC article. Review.
References
-
- Lee JH, Lee JE, Kahng JY, Kim SH, Park JS, Yoon SJ, Um JY, Kim WK, Lee JK, Park J, et al. Human glioblastoma arises from subventricular zone cells with low-level driver mutations. Nature. 2018;560(7717):243–7. - PubMed
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–66. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

