Functional insights from recombinant production of bacterial proteases in Saccharomyces cerevisiae
- PMID: 40405239
- PMCID: PMC12096733
- DOI: 10.1186/s12934-025-02732-x
Functional insights from recombinant production of bacterial proteases in Saccharomyces cerevisiae
Abstract
Background: Proteases are important enzymes in food and pharmaceutical industries, but challenges persist in their recombinant production due to host cell proteome hydrolysis and fitness loss. The development of recombinant expression systems for directed evolution of proteolytic enzymes, and industrial production are desirable. This study evaluated Saccharomyces cerevisiae as expression host for three bacterial proteases: BdpK (from Bdellovibrio bacteriovorus), IdeS, and SpeB (both from Streptococcus pyogenes), each with distinct peptide substrate scopes.
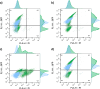
Results: We developed an experimental pipeline for analysis of protease gene expression levels and fitness effects on yeast cultures. Heterologous genes were fused with green fluorescent protein and their expression and effects on cell viability was monitored at the single-cell level by flow cytometry. IdeS-GFP fusion was produced efficiently with a gaussian distribution within the population and without compromising cell growth or viability. BdpK, on the other hand, displayed lower expression level and a more heterogenous distribution that was less stable over time. Production of SpeB was not feasible. Inserting the speB-GFP fusion gene resulted in complete growth inhibition and a significantly higher frequency of cells with compromised membrane integrity. Plasmid-based expression was compared with integrated-based expression, revealing higher total expression levels and lower degree of population heterogeneity for the latter.
Conclusions: S. cerevisiae was found to be an efficient expression host for the bacterial protease IdeS. In contrast, the expression of BdpK and SpeB faced significant challenges, including lack of activity for BdpK, or imposing a substantial fitness burden on the cells for SpeB, likely due to its broad substrate scope resulting in native protein degradation. The findings of this study provide valuable insights into the limitations and possibilities of yeast as an expression host for bacterial protease production and for studying their physiological effects using yeast as a model eukaryote.
Keywords: Saccharomyces cerevisiae host; Bacterial protease engineering; CRISPR/Cas9 integration; Protease expression; Recombinant enzyme production; Yeast expression system.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: TL was employed by Genovis AB during the preparation of this manuscript and RL is currently employed by Genovis AB. The authors declare no other competing interests.
Figures


References
-
- Ward OP. 3.49 - Proteases. Comprehensive Biotechnology (Second Edition). M. Moo-Young. Burlington, Academic Press: 2011; 571–582.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

