Novel fully human high-affinity anti-TREM2 antibody shows efficacy in clinically relevant Alzheimer´s mouse model
- PMID: 40405265
- PMCID: PMC12096560
- DOI: 10.1186/s13195-025-01759-x
Novel fully human high-affinity anti-TREM2 antibody shows efficacy in clinically relevant Alzheimer´s mouse model
Abstract
Background: New drugs to treat Alzheimer´s disease (AD) are urgently needed. Human triggering receptor expressed on myeloid cells 2 (hTREM2) is a validated drug target which is genetically associated with AD. Existing anti-hTREM2 antibodies were raised in animal immune systems, and subsequently humanized, which may incur immunological complications upon repeated preventive or therapeutic applications in vivo in AD patients. In addition, anti-hTREM2 antibodies should be optimized for both, efficacy and safety.
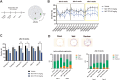
Methods: A novel fully human monoclonal brain-targeting anti-hTREM2 antibody M07-TFN was created. Binding affinities, cell viabilities, and agonist potencies were investigated on rhTREM2 and in human microglia. Transcytosis assays modeled blood-brain barrier translocation (BBB). Behavior tests were carried out in 5 × familiar AD (5xFAD) mice of both genders, to test for brain function and cognition as well as hippocampus-dependent spatial memory using the Barnes maze. In addition, amyloid plaque formation was determined on brain sections at the end of the study.
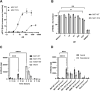
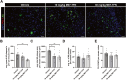
Results: M07-TFN showed higher binding affinities and stronger activation of hTREM2 signaling than all previously described anti-hTREM2 antibodies. p-Syk activation was increased 30-fold in hTREM2-overexpressing HEK293 cells and fourfold in human microglia cells compared to baseline. Human microglia viability significantly improved after stress testing. M07-TFN showed strong BBB translocation in a human BBB model, and exerted cross-reactivity to the mouse TREM2 stalk region, which allowed us to investigate M07-TFN directly in an AD mouse model. In 5xFAD mice, M07-TFN resulted in improved novel object location and better spatial orientation and memory, and significantly reduced plaque load. Additional safety investigations in mice showed no negative effects on blood cells or major organs.
Conclusion: Compared to existing humanized anti-hTREM2 antibodies that have been investigated in clinical trials, M07-TFN showed best-in-class affinities and agonist potencies. Being a fully human anti-hTREM2 antibody, M07-TFN holds the promise of reduced immunogenicity for use in human patients.
Keywords: Alzheimer’s disease; Antibody; Microglia; Neurodegeneration; TREM2.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments were permitted by the local Ethics board in accordance with European and German animal welfare legislations (5.1–231 5682/LMU/BMC/CAM). The most recent version of the Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines was strictly followed. Consent for publication: Not applicable. Competing interests: Several authors are employees of the biotech company ISAR Bioscience which is, however, owned (100%) by a non-profit foundation.
Figures

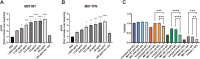



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

