Docosahexaenoic acid (DHA) supplementation attenuates changes in the concentration, phenotype, and response of immune peripheral blood cells in breast cancer patients undergoing neoadjuvant therapy. Secondary findings from the DHA-WIN trial
- PMID: 40405290
- PMCID: PMC12100857
- DOI: 10.1186/s13058-025-02048-z
Docosahexaenoic acid (DHA) supplementation attenuates changes in the concentration, phenotype, and response of immune peripheral blood cells in breast cancer patients undergoing neoadjuvant therapy. Secondary findings from the DHA-WIN trial
Abstract
Background: Breast cancer neoadjuvant therapy may negatively impact the immune system. As a secondary outcome of the docosahexaenoic acid (DHA) for women with breast cancer in the neoadjuvant setting (DHA-WIN trial), we sought to assess the effects of an intervention with DHA on parameters of immune function of women undergoing neoadjuvant therapy.
Methods: Women with early-stage breast cancer in the neoadjuvant setting were recruited for the DHA-WIN trial and randomly assigned to receive either 4.4 g/day of DHA or a placebo for 18 weeks in conjunction with their neoadjuvant chemotherapy for breast cancer. Venous blood was collected to isolate peripheral blood mononuclear cells. Immune parameters were assessed by measuring white blood cell concentration, flow cytometry, and cytokines concentration after mitogen-stimulated immune response.
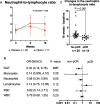
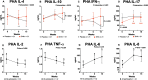
Results: In the placebo group the proportion of T cells (CD3 +), and functionally active monocytes (CD14 + HLA-DR +) was reduced at the last cycle of chemotherapy (15 weeks) but remained constant in the DHA group (P interaction < 0.05). The neutrophil-to-lymphocyte ratio (NLR) was maintained in the DHA group but increased in the placebo at the end of chemotherapy (P-interaction = 0.02). An increase in this ratio was associated with lower chance of achieving pathological complete response (OR = 0.32, 95% CI [0.14,0.16], P = 0.01). After 15 weeks of therapy, the DHA-supplemented group had higher concentrations of stimulated cytokines IL-4, IL-10, and the T helper type 1 cytokine IFN-γ after phytohemagglutinin (PHA) challenge, and higher concentrations of TNF-α and IFN-γ cytokines after lipopolysaccharide exposure (P < 0.05).
Conclusion: Supplementing DHA during breast cancer neoadjuvant chemotherapy improved systemic immune function by attenuating changes in blood cell concentrations, preventing depletion of immune cells, and enhancing ex vivo cytokine secretion after stimulation.
Keywords: Cytokines; Immune phenotype; Immune response; Long-chain polyunsaturated fatty acid; Pathological complete response.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Informed consent was obtained from all subjects involved in the study. Competing interests: The authors declare no competing interests.
Figures




References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. 10.3322/caac.21660. - PubMed
-
- Rubens RD, Sexton S, Tong D, Winter PJ, Knight RK, Hayward JL. Combined chemotherapy and radiotherapy for locally advanced breast cancer. Eur J Can. 1980;16(3):351–6. 10.1016/0014-2964(80)90352-7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

