ILOBONE: A phase I/IIa randomized controlled trial to assess the safety and feasibility of local iloprost therapy for enhancing proximal humerus fracture healing- a pilot study design
- PMID: 40405317
- PMCID: PMC12096472
- DOI: 10.1186/s13018-025-05865-2
ILOBONE: A phase I/IIa randomized controlled trial to assess the safety and feasibility of local iloprost therapy for enhancing proximal humerus fracture healing- a pilot study design
Abstract
Background: Proximal humerus fractures (PHFs) are the third most common fractures in elderly patients. Over 70% of PHFs in patients aged over 60 are displaced fractures, often necessitating surgical treatment. However, osteosynthesis is associated with a high rate of complications, highlighting the urgent need for additional therapeutic approaches to enhance bone healing and prevent osteonecrosis. This study evaluates the safety, feasibility and potential efficacy of local prostacyclin (iloprost) to improve bone healing in patients with PHFs.
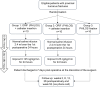
Methods: Thirty eligible patients will be randomized into one of three groups at a 1:1:1 ratio. All patients will receive angular stable locking plate fixation. Two treatment groups will receive an additional single dose of local iloprost through a 24-hour infusion postoperatively (group 1: low dose; group 2: high dose), while the control group will only receive the osteosynthesis. Patients will be monitored for 52 weeks. The primary endpoint is safety, with secondary endpoints including the preservation of the screw tip apex distance as an indicator of fracture healing, head shaft angle, necrosis rate, and patient-related outcome measures.
Discussion: The Ilobone study aims to provide data on the potential for biological augmentation of osteosynthesis procedures in PHFs, prone to healing challenges and complications.
Trial registration: The trial is registered with ClinicalTrial.gov (NCT04543682), registered 02 Sep. 2020, https://clinicaltrials.gov/show/NCT04543682 and the German Clinical Trials Registry (DRKS00027081), registered 10 Nov. 2021 https://drks.de/search/de/trial/DRKS00027081 .
Keywords: Bone healing; Fracture repair; Ilomedin; Iloprost; PHILOS; Proximal humerus fracture.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This study is being conducted in Germany. The recruiting center is located in Berlin at the Center for Musculoskeletal Surgery of the Charité– Universitaetsmedizin Berlin. The trial is conducted according to the ethical principles of the Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects, adopted by the General Assembly of the World Medical Association (1996), and is consistent with the ICH-GCP and applicable regulatory requirements. The trial sponsor is Charité– Universitaetsmedizin Berlin. Ethical approval was obtained from the ethics committee of Landesamt für Gesundheit und Soziales (LAGeSo) Berlin (Reference number 19/0132 - EK Mi) and was further approved by the Federal Institute for Drugs and Medical Devices (submission number: 4043778) (Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM)), which are the relevant competent authorities. Study amendments will be referred to the concerned Ethics Committee and/or competent authority (BfArM) for approval or notification, depending on the exact nature of the amendment. According to the new regulations, all amendments will be submitted through the Clinical Trials Information System (CTIS). Consent for publication: Not applicable. No identifying images or other personal or clinical details of the participants are presented here or will be presented in reports of the trial results. Competing interests: The authors declare no competing interests.
References
-
- Jones G, Nguyen T, Sambrook PN, Kelly PJ, Gilbert C, Eisman JA. Symptomatic fracture incidence in elderly men and women: the Dubbo Osteoporosis Epidemiology Study (DOES). Osteoporos Int [Internet]. 1994;4(5):277–82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7812076 - PubMed
-
- Palvanen M, Kannus P, Niemi S, Parkkari J. Update in the epidemiology of proximal humeral fractures. Clin Orthop Relat Res [Internet]. 2006;442:87–92. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16394745 - PubMed
-
- Neer CS. Displaced proximal humeral fractures. II. Treatment of three-part and four-part displacement. J Bone Joint Surg Am [Internet]. 1970;52(6):1090–103. Available from: http://www.ncbi.nlm.nih.gov/pubmed/5455340 - PubMed
-
- Neer CS. Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am [Internet]. 1970;52(6):1077–89. Available from: http://www.ncbi.nlm.nih.gov/pubmed/5455339 - PubMed
-
- Olerud P, Ahrengart L, Ponzer S, Saving J, Tidermark J. Internal fixation versus nonoperative treatment of displaced 3-part proximal humeral fractures in elderly patients: A randomized controlled trial. J Shoulder Elb Surg [Internet]. 2011;20(5):747–55. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21435907 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous