Comprehensive Profiling of Acral Lentiginous Melanoma Reveals Downregulated Immune Activation Compared to Cutaneous Melanoma
- PMID: 40405404
- PMCID: PMC12099029
- DOI: 10.1111/pcmr.70027
Comprehensive Profiling of Acral Lentiginous Melanoma Reveals Downregulated Immune Activation Compared to Cutaneous Melanoma
Abstract
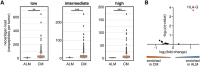
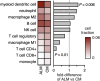
Acral lentiginous melanoma (ALM) is a rare and insufficiently understood subtype of melanoma lacking in effective treatment options. Recent work has demonstrated that the response of ALM to immune checkpoint blockade is inferior to that of cutaneous melanoma (CM). Here we performed bulk genomic and transcriptomic sequencing of tumor tissue from 28 ALM and 5692 CM cases. Similar to prior studies, ALM was associated with a significantly lower incidence of point mutations, including in the TERT promoter and BRAF, but increased numbers of gene amplifications, notably of CCND1, HMGA2, and MDM2. Reactome pathway analysis revealed enhancement of keratinization and PI3K/AKT signaling pathways. Overall immunogenicity was decreased in ALM, which possessed lower IFNγ (p < 0.001) and T-cell inflammatory (p = 0.03) pathway scores than CM. Despite higher computationally inferred levels of myeloid dendritic cells (p = 0.006), neoantigen load independent of predicted HLA binding affinity was lower (p < 0.01) in ALM versus CM. Assessment of classical and nonclassical HLA mRNA levels revealed upregulation of HLA-G, suggesting alternative ALM immune evasion pathways in the setting of lower PD-L1 expression (p = 0.005). Additional research is needed to better understand and therapeutically target signaling networks in the ALM tumor microenvironment.
Keywords: HLA‐G; acral lentiginous melanoma; multi‐omics; neoantigen load; tumor microenvironment.
© 2025 The Author(s). Pigment Cell & Melanoma Research published by John Wiley & Sons Ltd.
Conflict of interest statement
Joanne Xiu and Farah Abdulla are employed by Caris Life Sciences. Katherine M. Butcher has received travel support from Replimune and Iovance. Fumito Ito has consulted for Otsuka Pharmaceutical Factory Inc. Gino K. In has consulted for Pfizer and participated on advisory boards for BMS, Merck, Regeneron, Sanofi, Replimune, Novartis, and Pfizer. Institutional research support funds have been received from Regeneron, Idera, Replimune, Xencor, InstilBio, Pfizer, Obsidian, Bicara, Georgiamune Immunocore, and Checkmate Pharmaceuticals.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials