Early Results of the New Pamira ICD Lead
- PMID: 40405473
- PMCID: PMC12246506
- DOI: 10.1111/jce.16724
Early Results of the New Pamira ICD Lead
Abstract
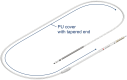
Introduction: A new implantable cardioverter-defibrillator (ICD) lead with a polyurethane cover added for improved long-term reliance was investigated in a post-market study.
Methods: The prospective BIO|MASTER Pamira study is designed to evaluate the new Pamira ICD lead in patients undergoing de-novo ICD or cardiac resynchronization therapy (CRT-D) implantation. We report first data from implantation and 6-month follow-up.
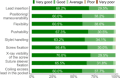
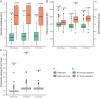
Results: Despite its slightly larger diameter, the lead demonstrated similar or improved handling during implantation compared to its predecessor without polyurethane cover.
Conclusion: All results from implantation and short-term follow-up aligned with expectations. To show an improved reliance, long-term data will be required.
Keywords: implantable cardioverter defibrillator; lead handling; lead implantation; right ventricular lead; safety.
© 2025 The Author(s). Journal of Cardiovascular Electrophysiology published by Wiley Periodicals LLC.
Conflict of interest statement
Nils Jenke is an employee of Biotronik. All clinical authors received patient fees in the context of the study, which were paid to their institutions.
Figures
References
-
- Al‐Khatib S. M., Stevenson W. G., Ackerman M. J., et al., “2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society,” Circulation 138 (2018): 210, 10.1161/CIR.0000000000000548. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

