Glut1 Deficiency Syndrome: Novel Pathomechanisms, Current Concepts, and Challenges
- PMID: 40405536
- PMCID: PMC12099281
- DOI: 10.1002/jimd.70044
Glut1 Deficiency Syndrome: Novel Pathomechanisms, Current Concepts, and Challenges
Abstract
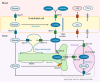
Glut1 Deficiency Syndrome (Glut1DS) has emerged as a treatable, but complex entity. Increasing data on pathogenic mechanisms, phenotype, genotype, and ketogenic dietary therapies (KDT) are available, as summarized in this review. Many challenges remain: novel symptoms emerge and vary with age. In Glut1DS, KDT in pregnancy and the clinical features in neonates and adults are poorly understood. KDT are ineffective in some patients for reasons yet unknown. Research reaches beyond the concept of brain energy depletion by impaired GLUT1-mediated glucose transfer across the blood-brain barrier. Novel concepts investigate alternative substrates, transport mechanisms, and metabolic interactions of different brain cell types. Future, yet currently unavailable prospects are neonatal screening for Glut1DS, reliable biomarkers, predictors for outcome, and alternative therapies, along with and beyond KDT.
Keywords: De Vivo disease; GLUT1; Glut1 Deficiency Syndrome; Glut1DS; SLC2A1; hypoglycorrhachia; ketogenic dietary therapies; treatable.
© 2025 The Author(s). Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
Joerg Klepper has sat on Conference Scientific Advisory Panels for Nutricia and Vitaflo for which remuneration has been paid. He has received travel expenses from Nutricia and Vitaflo for invited lectures and for the Clinical Training Fellowship in KDT (KetoCollege by Matthews Friends, UK). He is currently a board member of the International Neurological Ketogenic Society (INKS) as well as Clinical Advisor to US, UK, German, and Austrian Glut1DS parent support groups.
Figures


References
-
- De Vivo D. C., Trifiletti R. R., Jacobson R. I., Ronen G. M., Behmand R. A., and Harik S. I., “Defective Glucose Transport Across the Blood‐Brain Barrier as a Cause of Persistent Hypoglycorrhachia, Seizures, and Developmental Delay,” New England Journal of Medicine 325, no. 10 (1991): 703–709. - PubMed
-
- Wang D., Pascual J. M., and de Vivo D., “Glucose Transporter Type 1 Deficiency Syndrome,” in GeneReviews((R)), ed. Adam M. P., Feldman J., Mirzaa G. M., et al. (1993).
-
- Brockmann K., Wang D., Korenke C. G., et al., “Autosomal Dominant Glut‐1 Deficiency Syndrome and Familial Epilepsy,” Annals of Neurology 50, no. 4 (2001): 476–485. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

