Puumala orthohantavirus: prevalence, biology, disease, animal models and recent advances in therapeutics development and structural biology
- PMID: 40406115
- PMCID: PMC12095308
- DOI: 10.3389/fimmu.2025.1575112
Puumala orthohantavirus: prevalence, biology, disease, animal models and recent advances in therapeutics development and structural biology
Abstract
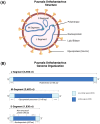
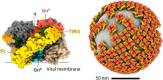
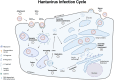
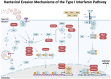
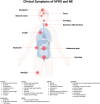
Puumala orthohantavirus (PUUV) is an emerging zoonotic virus that was first discovered in the Puumala region of Finland in the early 1980s and is the primary etiological agent of nephropathia epidemica (NE), a milder form of a life-threatening disease known as hemorrhagic fever with renal syndrome (HFRS). PUUV and other members of the Old World hantaviruses (OWHVs) predominantly circulate in rodents or insectivores across Eurasia, accounting for several thousand of reported HFRS cases every year (with many more unreported/misdiagnosed cases suspected). The rodent reservoir of PUUV is the common bank vole (Myodes (M.) glareolus), and transmission of the virus to humans occurs via inhalation of contagious aerosols and through contact with contaminated droppings or urine. Although PUUV is the subject of extensive research, due to its potential to cause severe disease outcomes in humans and its considerable economic and social impact, neither licensed vaccines nor specific antiviral treatments are available against PUUV. However, many important advancements have been made in terms of PUUV research over the last years. This included the elucidation of its glycoproteins, the discovery of broadly neutralizing hantavirus antibodies as therapeutic candidates and expanded research on the mRNA vaccine technology which will likely enable the development of strong PUUV vaccine candidates in the near future. Currently, there is still a lack of suitable animal models for the preclinical evaluation of experimental vaccines and antivirals, which hampers vaccine and antiviral development. Current attempts to decrease hantavirus-associated human infections rely primarily on prevention and countermeasures for rodent control, including reduced contact to droppings, saliva and urine, and disinfection of areas that are contaminated with rodent excreta. Here, we review these recent advances and other aspects including PUUV prevalence, virus biology, diagnosis and clinical features, and current animal models for vaccine and treatment development.
Keywords: Puumala orthohantavirus; animal models; antiviral treatment; glycoprotein; nephropathia epidemica; vaccine research.
Copyright © 2025 Tscherne, Guardado-Calvo, Clark, Krause and Krammer.
Conflict of interest statement
FK declares the following conflicts of interest. The Icahn School of Medicine at Mount Sinai has filed patent applications regarding influenza virus vaccines on which FK is listed as inventor. The Icahn School of Medicine at Mount Sinai has filed patent applications relating to SARS-CoV-2 serological assays, NDV-based SARS-CoV-2 vaccines influenza virus vaccines and influenza virus therapeutics which list FK as co-inventor and FK has received royalty payments from some of these patents. Mount Sinai has spun out a company, Kantaro, to market serological tests for SARS-CoV-2 and another company, Castlevax, to develop SARS-CoV-2 vaccines. FK is co-founder and scientific advisory board member of Castlevax. FK has consulted for Merck, GSK, Sanofi, Curevac, Seqirus and Pfizer and is currently consulting for 3rd Rock Ventures, Gritstone and Avimex. The Krammer laboratory is also collaborating with Dynavax on influenza vaccine development and with VIR on influenza virus therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

