Exogenous GM-CSF therapy for autoimmune pulmonary alveolar proteinosis: a systematic literature review
- PMID: 40406404
- PMCID: PMC12094945
- DOI: 10.3389/fmed.2025.1552566
Exogenous GM-CSF therapy for autoimmune pulmonary alveolar proteinosis: a systematic literature review
Erratum in
-
Corrigendum: Exogenous GM-CSF therapy for autoimmune pulmonary alveolar proteinosis: a systematic literature review.Front Med (Lausanne). 2025 Jun 3;12:1632193. doi: 10.3389/fmed.2025.1632193. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40529121 Free PMC article.
Abstract
Background: Granulocyte-macrophage colony-stimulating factor (GM-CSF) therapy is an important treatment for autoimmune pulmonary alveolar proteinosis (aPAP). Exogenous GM-CSF treatment can be administered either through subcutaneous injection or nebulized inhalation. However, data on the effectiveness and safety of these two approaches are lacking.
Method: We conducted a systematic literature review of different methods, including subcutaneous injection and nebulized inhalation of GM-CSF, for the treatment of aPAP patients. Patients were divided into a subcutaneous injection group (SIG) and a nebulized inhalation group (NIG) according to the route of administration. Treatment efficacy and safety, including adverse events, were statistically assessed. We analyzed different GM-CSF treatment cycles with different time intervals. The analyses were performed using chi-square tests, unpaired t-tests, and Kruskal-Wallis H-tests.
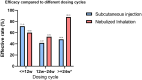
Results: A total of 304 aPAP patients were treated with GM-CSF, including 66 (21.7%) in the SIG and 238 (78.3%) in the NIG. In total, we identified 220 (72.37%) patients whose treatment was effective and 84 (27.63%) patients whose treatment was ineffective. Efficacy was achieved in 54.55% (36/66) of the SIG patients and 77.31% (184/238) of the NIG patients (P < 0.001). More metrics were changed than in the NIG than SIG, suggesting the superior effectiveness of nebulized inhalation. The nebulized inhalation of GM-CSF was more effective (P < 0.001) and caused fewer adverse events than its subcutaneous injection. A significant difference in the NIG was noted across treatment durations, with an efficacy rate of 88% for those treated for over 24 weeks, compared with 48% in the SIG (P < 0.001). Among the NIG patients, the optimal efficacy was found to be at a dosage of 300-400 μg/d, with diminishing efficacy at higher doses (P < 0.036).
Conclusion: Nebulized inhalation is a more effective and safer route of GM-CSF administration than subcutaneous injection is, with a potential optimal dosage of 300-400 μg/day, and the duration of GM-CSF treatment via nebulized inhalation with the greatest efficacy is >24 weeks.
Keywords: GM-CSF; aPAP; inhalation; subcutaneous injection; treatment dosage and duration.
Copyright © 2025 Chen, Feng, Yao, Li, Yang, Qin, Li and Qiu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

