Vancomycin dosing in neonates: enhancing outcomes using population pharmacokinetics and simulation
- PMID: 40406542
- PMCID: PMC12095254
- DOI: 10.3389/frabi.2025.1568931
Vancomycin dosing in neonates: enhancing outcomes using population pharmacokinetics and simulation
Abstract
Introduction: Optimizing vancomycin dosing in neonates is a critical yet complex goal. Traditional trough concentration-based dosing strategies correlate poorly with therapeutic efficacy and often fail to account for the significant renal function variability and drug clearance in neonates. The 24-hour area under the concentration-time curve to minimum inhibitory concentration (AUC24/MIC) ≥ 400 mg h/L has emerged as a superior pharmacodynamic target. Population pharmacokinetics (PopPK) models allow optimized dosing by incorporating neonatal-specific factors such as postmenstrual age (PMA), gestational age (GA), serum creatinine (SCr), and weight.
Objective: To develop optimized vancomycin dosing regimens for neonates that achieve an 80% probability of target attainment (PTA) for an AUC24/MIC ≥ 400 mg h/L across diverse clinical cohorts and simulated neonatal populations.
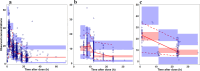
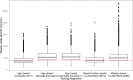
Methods: Real-world data from three international centers (Belgium, New Zealand, USA), including 610 individuals and 2399 vancomycin concentrations, were used to externally evaluate a previously published PopPK model (NONMEM®). Missing data, including body weight, were imputed using Amelia II version 1.7.3 for R, while Zelig for R integrated multiple imputed datasets. A virtual population of 10,000 neonates was independently generated using MATLAB to simulate clinical scenarios considering covariates such as PMA, GA, SCr, body weight, and imputed body length.
Results: Simulations showed that PMA and SCr were key covariates that significantly improved PTA, particularly in preterm neonates. Preterm neonates achieved PTAs of 80% with daily doses of 30 or 40 mg/kg/day, while term neonates required 15 mg/kg every 8 hours or 20 mg/kg every 12 hours. The simulations demonstrated that these optimized dosing strategies achieved an 80% PTA for AUC24/MIC ≥ 400 mg h/L in the virtual neonatal population. For neonates with PMA < 29 weeks and SCr > 0.6 mg/dL, including SCr as a covariate increased the likelihood of achieving the target from 65% to 87%.
Conclusion: Incorporating developmental factors like PMA and SCr into vancomycin dosing strategies achieved robust and clinically relevant outcomes. The optimized regimens achieved an 80% PTA for the AUC24/MIC target for preterm and term neonates. These findings offer a scalable framework for improving neonatal vancomycin pharmacotherapy across diverse populations and clinical settings.
Keywords: dosing regimens; neonatology; population pharmacokinetics; simulations; the probability of target attainment; vancomycin.
Copyright © 2025 Illamola, Bhongsatiern, Birnbaum, Kumar, Courter, Haslam, Allegaert, Reith, Desai and Sherwin.
Conflict of interest statement
JJB was supported by a Scholarship during the study. JJB is employed by Regeneron Pharmaceuticals Inc. SSK is employed by Parexel International, Sydney, Australia, However, SKK contributed to this article as an employee of University of Utah School of Medicine. CMS is employed by Differentia Bio; the work was undertaken while CMS was employed by University of Utah School of Medicine. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures


References
-
- Ainsworth D. S. B. (2014). Neonatal Formulary 7: Drug Use in Pregnancy and the First Year of Life (Chichester, UK: John Wiley & Sons, Ltd; ), 520–522.
-
- American Academy of Pediatrics (2009). “Staphylococcal infections,” in Red Book 2009 Report of the Committee on Infection Diseases. Eds. Pickering L. K., Baker C. J., Kimberlin D. W., Long S. S. (American Academy of Pediatrics, Elk Grove Village, IL: ), 601–615.
-
- American Academy of Pediatrics (2012). “Staphylococcal infections,” in Red Book 2012 Report of the committee on infectious diseases, 29 ed. Eds. Pickering L. K., Baker C. J., Kimberlin D. W., Long S. S. (American Academy of Pediatrics, Elk Grove Village, IL: ), 652–821.
LinkOut - more resources
Full Text Sources

