Disruption of ATR Signaling by Epstein-Barr Virus Latent Membrane Protein 1 Sensitizes Nasopharyngeal Carcinoma Cells to Cisplatin
- PMID: 40407079
- PMCID: PMC12100692
- DOI: 10.1002/jmv.70407
Disruption of ATR Signaling by Epstein-Barr Virus Latent Membrane Protein 1 Sensitizes Nasopharyngeal Carcinoma Cells to Cisplatin
Abstract
Nasopharyngeal carcinoma (NPC) occurs with high incidence in Southeast Asia where almost all tumors are associated with Epstein-Barr virus (EBV) infection. Cisplatin is used in combination chemotherapy. In this study, we determined that the EBV oncoprotein, latent membrane protein 1 (LMP1), perturbs DNA damage response (DDR) signaling, activation of cell cycle checkpoints, and sensitivity to cisplatin in NPC cells (HK1). Hypersensitivity was validated by LMP1 knockdown and CRISPR/Cas9 targeting in HK1-EBV cells with latent EBV infection. The conserved PxQxT motif (in CTAR1) and Y384 residue (in CTAR2) were required for the hypersensitivity. Inhibition of ATR (VE821 or AZD6738), but not ATM (KU55933 or AZD0156), phenocopied the G1 arrest and hypersensitivity. Attenuation of DDR signaling and hypersensitivity by LMP1 or ATR inhibition was also observed in the C17 NPC cell line with restored stable LMP1 expression. LMP1 expression in NPC tumors is highly variable. Publicly available RNA-sequencing data from microdissected NPC tumors showed that LMP1 expression in the primary tumors was the lowest in cisplatin-treated patients that experienced recurrence. These findings could have clinical significance in stratifying NPC patients such that tumors with limited or variable LMP1 expression might benefit from ATR inhibitor therapy.
Keywords: DNA damage response signaling; Epstein–Barr virus; latent membrane protein 1; nasopharyngeal carcinoma.
© 2025 The Author(s). Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
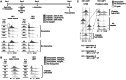
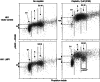






References
-
- Pathmanathan R., Prasad U., Sadler R., Flynn K., and Raab‐Traub N., “Clonal Proliferations of Cells Infected With Epstein‐Barr Virus in Preinvasive Lesions Related to Nasopharyngeal Carcinoma,” New England Journal of Medicine 333, no. 11 (1995): 693–698. - PubMed
-
- Yip Y. L., Tsang C. M., Deng W., et al., “Expression of Epstein‐Barr Virus‐Encoded LMP1 and hTERT Extends the Life Span and Immortalizes Primary Cultures of Nasopharyngeal Epithelial Cells,” Journal of Medical Virology 82, no. 10 (2010): 1711–1723. - PubMed
-
- Lo K. W., To K. F., and Huang D. P., “Focus on Nasopharyngeal Carcinoma,” Cancer Cell 5, no. 5 (2004): 423–428. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

