TLR3 mediates central sensitization in a chronic migraine model induced by repeated nitroglycerin through the ERK signaling pathway
- PMID: 40407181
- PMCID: PMC12185963
- DOI: 10.1177/17448069251346373
TLR3 mediates central sensitization in a chronic migraine model induced by repeated nitroglycerin through the ERK signaling pathway
Abstract
Background: Studies have demonstrated that Toll-like receptor 3 (TLR3) plays a crucial role in neuropathic pain. However, there have been no relevant reports regarding the role of TLR3 in migraine chronification. This study aims to investigate the molecular mechanisms of TLR3 in the central sensitization of chronic migraine (CM).
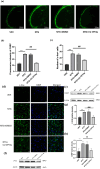
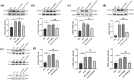
Methods: C57BL/6 male mice were used as models for chronic migraine (CM) disease, receiving an intraperitoneal injection of nitroglycerin (NTG) every other day. Calibrated von Frey filaments were employed to measure the pain threshold in the hind paw sole and periorbital region, enabling the assessment of mechanical allodynia. Western blot was employed to detect the expression changes of TLR3, TRAF6, TAK1, c-Fos, calcitonin gene-related peptide (CGRP), and the extracellular signal-regulated kinase (ERK) signaling pathway. Immunofluorescence was used to detect the cellular localization of TLR3 and the expression changes of central sensitization-related indicators, such as c-Fos and CGRP. In addition, we investigated the effects of TLR3 inhibitor (CU CPT4a), MEK inhibitor(PD98059), TRAF6 inhibitor(C25-140), and TAK1 inhibitor (Takinib) on chronic migraine-like behavior, and activation of the ERK pathway in the Trigeminal nucleus caudalis (TNC).
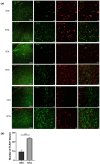
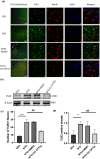
Results: Recurrent injections of NTG resulted in a significant increase in the expression of TLR3, TRAF6, TAK1, CGRP, and c-Fos proteins, as well as the activation of the ERK signaling pathway. Concurrent inhibition of TLR3 function, TRAF6, TAK1, and the ERK pathway counteracted these changes and alleviated hyperalgesia in CM mice.
Conclusions: Our findings suggest that TLR3 may play a role in central sensitization in CM mice by TRAF6-TAK1 axis modulating the ERK signaling pathway.
Keywords: ERK; TLR3; central sensitization; chronic migraine; neuron.
Conflict of interest statement
Declaration of conflicting interestsThe author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures






Similar articles
-
Chronification of migraine sensitizes to CGRP in male and female mice.Cephalalgia. 2025 Feb;45(2):3331024251317446. doi: 10.1177/03331024251317446. Cephalalgia. 2025. PMID: 39945018 Free PMC article.
-
Microglial NLRP3 inflammasome activation mediates IL-1β release and contributes to central sensitization in a recurrent nitroglycerin-induced migraine model.J Neuroinflammation. 2019 Apr 10;16(1):78. doi: 10.1186/s12974-019-1459-7. J Neuroinflammation. 2019. PMID: 30971286 Free PMC article.
-
TLR2 Mediates Microglial Activation and Contributes to Central Sensitization in a Recurrent Nitroglycerin-induced Chronic Migraine Model.Mol Neurobiol. 2024 Jun;61(6):3697-3714. doi: 10.1007/s12035-023-03781-2. Epub 2023 Nov 27. Mol Neurobiol. 2024. PMID: 38008889
-
Preventive drug treatments for adults with chronic migraine: a systematic review with economic modelling.Health Technol Assess. 2024 Oct;28(63):1-329. doi: 10.3310/AYWA5297. Health Technol Assess. 2024. PMID: 39365169 Free PMC article.
-
Botulinum toxins for the prevention of migraine in adults.Cochrane Database Syst Rev. 2018 Jun 25;6(6):CD011616. doi: 10.1002/14651858.CD011616.pub2. Cochrane Database Syst Rev. 2018. PMID: 29939406 Free PMC article.
References
-
- Stovner L, Hagen K, Jensen R, Katsarava Z, Lipton R, Scher A, Steiner T, Zwart JA. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia 2007; 27: 193–210. - PubMed
-
- Schwedt TJ. Chronic migraine. BMJ 2014; 348: g1416. - PubMed
-
- Dodick DW. Migraine. Lancet 2018; 391: 1315–1330. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

