Inhibiting FAT1 Blocks Metabolic Bypass to Enhance Antitumor Efficacy of TCA Cycle Inhibition through Suppressing CPT1A-Dependent Fatty Acid Oxidation
- PMID: 40407216
- PMCID: PMC12376706
- DOI: 10.1002/advs.202502146
Inhibiting FAT1 Blocks Metabolic Bypass to Enhance Antitumor Efficacy of TCA Cycle Inhibition through Suppressing CPT1A-Dependent Fatty Acid Oxidation
Abstract
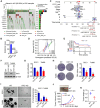
FAT atypical cadherin 1 (FAT1) is one of the most frequently mutated genes in head and neck squamous cell carcinoma (HNSCC), exhibiting the highest mutation rate across different tumor types. Although FAT1's role has attracted considerable attention, its impact on cancer metabolism and treatment resistance remains poorly understood. In this study, it is demonstrated that knockout of mutant FAT1 in HNSCC cells attenuates CPT1A-driven fatty acid oxidation (FAO) through downregulation of the transcription factor ASCL2, leading to marked suppression of tumor growth. Notably, FAT1-mutant HNSCC cells exhibit resistance to the TCA cycle inhibitor CPI-613 through activation of CPT1A-mediated FAO, whereas genetic ablation of mutant FAT1 restores sensitivity to CPI-613. To achieve in vivo depletion of FAT1, LNP-sgFAT1 is developed, a novel lipid nanoparticle (LNP) system encapsulating Cas9 mRNA and FAT1-targeting sgRNA. In murine models bearing FAT1-mutant head and neck tumors, LNP-sgFAT1 demonstrated enhanced antitumor activity when combined with CPI-613. Collectively, these findings establish that mutant FAT1 drives CPT1A-dependent FAO, facilitating a metabolic bypass that confers resistance to TCA cycle inhibition in HNSCC. This mechanistic insight highlights promising opportunities for combinatorial therapeutic strategies co-targeting genetic and metabolic vulnerabilities in cancer.
Keywords: CPI‐613 sensitivity; CPT1A; FAT1; fatty acid oxidation; head and neck cancer; metabolic bypass.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
YT has previously received funds for research contracts from Cornerstone Pharmaceuticals. NFS reports compensated and uncompensated advisory roles with: Astra Zeneca, Eisai Medical, Exelixis, Merck, Merck EMD Serono, Pfizer, Kura, Vaccinex, CUE, BionTech, GSK, TOSK, Seagen, Flamingo, Infinity, Inovio, Aveo, Medscape, Onclive, Uptodate, BMS, Cornerstone, Celldex, Surface Oncology, Astex, Imugene, Faron Pharmaceutical, Coherus, Adagene, Fulgent Springer, Nanobiotix, and Taiho; funding from: Exelixis, BMS. The other authors declare no conflict of interest.
Figures






References
-
- Saba N. F., Steuer C. E., Ekpenyong A., McCook‐Veal A., Magliocca K., Patel M., Schmitt N. C., Stokes W., Bates J. E., Rudra S., Remick J., McDonald M., Abousaud M., Tan A. C., Fadlullah M. Z. H., Chaudhary R., Muzaffar J., Kirtane K., Liu Y., Chen G. Z., Shin D. M., Teng Y., Chung C. H., Nat. Med. 2023, 29, 880. - PMC - PubMed
-
- Siegel R. L., Miller K. D., Jemal A., CA Cancer J. Clin. 2020, 70, 7. - PubMed
-
- Shin D. M., Khuri F. R., Head Neck 2013, 35, 443. - PubMed
-
- Carlisle J. W., Steuer C. E., Owonikoko T. K., Saba N. F., CA: Cancer J. Clin. 2020, 70, 505. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
