Memory T Cells in Respiratory Virus Infections: Protective Potential and Persistent Vulnerabilities
- PMID: 40407543
- PMCID: PMC12101432
- DOI: 10.3390/medsci13020048
Memory T Cells in Respiratory Virus Infections: Protective Potential and Persistent Vulnerabilities
Abstract
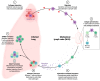
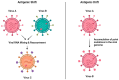
Respiratory virus infections, such as those caused by influenza viruses, respiratory syncytial virus (RSV), and coronaviruses, pose a significant global health burden. While the immune system's adaptive components, including memory T cells, are critical for recognizing and combating these pathogens, recurrent infections and variable disease outcomes persist. Memory T cells are a key element of long-term immunity, capable of responding swiftly upon re-exposure to pathogens. They play diverse roles, including cross-reactivity to conserved viral epitopes and modulation of inflammatory responses. However, the protective efficacy of these cells is influenced by several factors, including viral evolution, host age, and immune system dynamics. This review explores the dichotomy of memory T cells in respiratory virus infections: their potential to confer robust protection and the limitations that allow for breakthrough infections. Understanding the underlying mechanisms governing the formation, maintenance, and functional deployment of memory T cells in respiratory mucosa is critical for improving immunological interventions. We highlight recent advances in vaccine strategies aimed at bolstering T cell-mediated immunity and discuss the challenges posed by viral immune evasion. Addressing these gaps in knowledge is pivotal for designing effective therapeutics and vaccines to mitigate the global burden of respiratory viruses.
Keywords: adaptive immunity; immunology; lymphocyte; memory T cells; viral infection.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Richter B.W.M., Onuska J.M., Niewiesk S., Prince G.A., Eichelberger M.C. Antigen-Dependent Proliferation and Cytokine Induction in Respiratory Syncytial Virus-Infected Cotton Rats Reflect the Presence of Effector-Memory T Cells. Virology. 2005;337:102–110. doi: 10.1016/j.virol.2005.04.001. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

