A cost-effectiveness analysis comparing single-inhaler extrafine beclomethasone/formoterol/glycopyrronium bromide against other SITTs in adult patients with uncontrolled asthma in England
- PMID: 40407861
- PMCID: PMC12100855
- DOI: 10.1186/s13561-025-00640-9
A cost-effectiveness analysis comparing single-inhaler extrafine beclomethasone/formoterol/glycopyrronium bromide against other SITTs in adult patients with uncontrolled asthma in England
Abstract
Background: In patients with asthma uncontrolled by a medium or high-strength (MS/HS) inhaled corticosteroid (ICS) plus long-acting β2-agonist (LABA), according to Global Initiative for Asthma (GINA) guidelines, a maintenance therapy option is the addition of a long-acting muscarinic antagonist (LAMA) via single-inhaler triple therapy (SITT). Evidence has previously been published on the cost-effectiveness of a SITT extra fine formulation of beclomethasone, formoterol and glycopyrronium bromide (BDP/FOR/GLY) vs. dual ICS/LABA combination, using data from two 52-week clinical trials (TRIMARAN and TRIGGER). However, there is limited evidence on the comparative cost-effectiveness of SITTs. The current analysis evaluated the cost-effectiveness of BDP/FOR/GLY versus other SITTs, in the UK setting.
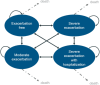
Methods: Markov cohort state-transition model was developed to investigate the cost-effectiveness of BDP/FOR/GLY Medium Strength (MS) vs. fluticasone, umeclidinium, and vilanterol (FF/UMEC/VI) MS and, BDP/FOR/GLY High Strength vs. FF/UMEC/VI HS and vs. indacaterol acetate, glycopyrronium bromide, and mometasone (IND/GLY/MF) HS. A network meta-analysis was performed to estimate comparative efficacy of BDP/FOR/GLY against other SITTs. The model analyzed cost, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratio (ICER), net monetary benefit (NMB), and was developed from the perspective of England National Health Service (NHS) and Prescribed Specialized Services expenditure (2022 costs). Uncertainty of the inputs was estimated using one-way and probabilistic sensitivity analyses.
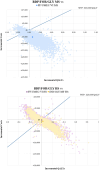
Results: BDP/FOR/GLY MS was projected to be a dominant treatment alternative against FF/UMEC/VI MS (£5,121 less costly, gained 0.065 additional QALYs). Similarly, BDP/FOR/GLY HS was a dominant treatment alternative against FF/UMEC/VI HS (£143, 0.003 additional QALYs) and IND/GLY/MF HS (£692 less costly, gained 0.023 additional QALYs). BDP/FOR/GLY MS and HS had 77.1%, 51.3%, and 61.2% likelihoods to be cost-effective vs. FF/UMEC/VI MS, FF/UMEC/VI HS, and IND/GLY/MF HS at the defined willingness-to-pay (WTP) threshold of £20,000 per QALY gained, respectively.
Conclusions: BDP/FOR/GLY MS and HS were a dominant treatment alternative compared with FF/UMEC/VI, both MS and HS, and IND/GLY/MF HS in patients with asthma uncontrolled by ICS/LABA.
Keywords: Asthma; Cost-effectiveness; England; Exacerbations; Network meta-analysis; Single-inhaler triple therapy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Previous presentations: The paper is original, nor all neither any of its parts have been reproduced in other publications.
Figures

References
-
- GINA. Global strategy for asthma management and prevention. 2024.
-
- World Health Organization. Asthma: key facts. 2023 30 Nov 2023]; Available from: https://www.who.int/news-room/fact-sheets/detail/asthma
-
- National Institute for Health and Care Excellence (NICE). Prevalence and background information on asthma. 2023 30 Nov 2023]; Available from: https://www.nice.org.uk/cks-uk-only#:~:text=Prognosis-,What is the preva...
-
- UptoDate. An overview of asthma management. 2023 4 Dec 2023]; Available from: https://www.uptodate.com/contents/an-overview-of-asthma-management
LinkOut - more resources
Full Text Sources

