Cardiac-specific troponin-I (cTnI) in a post-mortem setting
- PMID: 40407876
- PMCID: PMC12354124
- DOI: 10.1007/s00414-025-03526-x
Cardiac-specific troponin-I (cTnI) in a post-mortem setting
Abstract
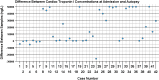
Cardiac-specific troponin (cTn) is widely used in clinical medicine to support a diagnosis of acute myocardial infarction. Several studies have explored the value of cTn testing in deceased individuals. These studies suggest that -although there are important limitations associated with its use- post-mortem cTn can be useful in selected cases. A decision for post-mortem cTn testing should however be influenced by factors that have not been explored in much detail. This includes the success rate of post-mortem cTn testing, and whether cTn levels are stable after death.Therefore, this study addresses the post-mortem availability and stability of cardiac-specific Troponin I (cTnI). Post-mortem availability was determined by analysing the success rate in 250 high-sensitivity (hs-)cTnI tests on post-mortem blood samples, and its relationship with variables such as sample location, sample type, post-mortem interval, and decomposition. Post-mortem stability was explored by comparing post-mortem cTnI levels between two samples from the same individual, taken at different times.Post-mortem hs-cTnI tests were successful in 86.4% of cases (216/250), with little effect of sex, age, or cardiopulmonary resuscitation. Visible decomposition precluded a successful test. Other variables associated with decomposition (such as increased post-mortem interval) also affected test success negatively. Our results furthermore suggest that cTnI is very unstable post-mortem, with marked differences in hs-cTnI test results between samples from the same individual. The differences were large (on average 18734 ng/L) and not unidirectional. Instability appeared to increase with larger time intervals, but the results were overall erratic and difficult to interpret.We conclude that hs-cTnI testing results are generally available in a post-mortem setting, but that testing should be performed on the earliest available blood sample. Samples from decomposed individuals should not be tested. Furthermore, the severe instability of cTnI indicates that any post-mortem hs-cTnI result must be interpreted with caution.
Keywords: Autopsy; Biochemistry; Blood analysis; Decomposition; Forensic medicine; Pathology; Sudden cardiac death; Troponin; cTnI.
© 2025. Crown.
Conflict of interest statement
Declarations. Human ethics approval: This study was approved by the Victorian Institute of Forensic Medicine (VIFM) ethics committee on the 26th of October 2022 (Project ID: 1247). This study was conducted in accordance with the Declaration of Helsinki. Clinical trial number: not applicable.
Figures
References
-
- Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA (2022) The global burden of cardiovascular diseases and risk: A compass for future health. J Am Coll Cardiol 80:2361–2371. 10.1016/j.jacc.2022.11.005 - PubMed
-
- Zeppenfeld K, Tfelt-Hansen J, de Riva M et al (2022) 2022 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J 43:3997–4126. 10.1093/eurheartj/ehac262 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

