Health-related quality of life with daratumumab, bortezomib, melphalan, and prednisone versus bortezomib, melphalan, and prednisone alone in transplant-ineligible patients with newly diagnosed multiple myeloma: analysis of the phase 3 OCTANS study
- PMID: 40407887
- PMCID: PMC12141397
- DOI: 10.1007/s00277-025-06303-3
Health-related quality of life with daratumumab, bortezomib, melphalan, and prednisone versus bortezomib, melphalan, and prednisone alone in transplant-ineligible patients with newly diagnosed multiple myeloma: analysis of the phase 3 OCTANS study
Abstract
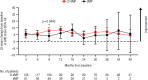
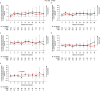
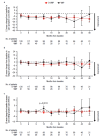
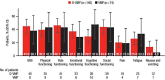
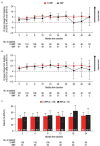
In the phase 3 OCTANS study, daratumumab plus bortezomib/melphalan/prednisone (D-VMP) significantly improved response rates and progression-free survival versus VMP alone in transplant-ineligible Asian patients with newly diagnosed multiple myeloma (NDMM). Understanding the impact of treatment on patient-reported outcomes (PROs) is of increasing interest. Here, we report on the impact of D-VMP and VMP on PROs in OCTANS. PROs were a secondary endpoint and were assessed using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30-item (EORTC QLQ-C30) and the EuroQol 5-dimensional descriptive system (EQ-5D-5L) questionnaire, administered at screening, every 3 months (Year 1), every 6 months thereafter (until disease progression), and 8 and 16 weeks post disease progression. Treatment effects were estimated using a mixed-effects model with repeated measures. Overall, 220 patients were randomized (D-VMP, n = 146; VMP, n = 74). Compliance rates were 100% at baseline in both treatment groups and remained relatively high by Month 12 (D-VMP, 82.6%; VMP, 67.4%). Comparable improvements from baseline were generally observed between treatment groups across most PRO scores. Significant improvements were observed with D-VMP versus VMP in Global Health Status (GHS) score at 9 months (p = 0.0443) and in social functioning and nausea and vomiting symptom scores at 12 months (p = 0.0042 and p = 0.0012, respectively). In summary, transplant-ineligible Asian patients with NDMM demonstrated improvements in PROs following treatment with D-VMP and VMP; however, greater improvements were observed in GHS, social functioning, and nausea and vomiting symptoms with D-VMP.
ClinicalTrials.gov Identifier: NCT03217812; submitted July 13, 2017.
Trial registration: ClinicalTrials.gov NCT03217812.
Keywords: Daratumumab; Multiple myeloma; Patient-reported outcomes; Transplant-ineligible.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: The study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki, Good Clinical Practices, and abided by all applicable regulatory requirements. The study protocol and amendments were reviewed and approved by Independent Ethics Committees and Institutional Review Boards at each participating site, and all patients provided written informed consent. Competing interests: R.C., R.L., X.C., and C.C. are employees of Johnson & Johnson and own stock or stock options. M.Z. is an employee of IQVIA, which was contracted by Johnson & Johnson for the presented analyses. J.W. served as a member of the board of directors or advisory committees at AbbVie. All other authors have nothing to declare.
Figures
References
-
- de Weers M, Tai YT, van der Veer MS, Bakker JM, Vink T, Jacobs DC, Oomen LA, Peipp M, Valerius T, Slootstra JW, Mutis T, Bleeker WK, Anderson KC, Lokhorst HM, van de Winkel JG, Parren PW (2011) Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J Immunol 186:1840–1848. 10.4049/jimmunol.1003032 - PubMed
-
- Lammerts van Bueren J, Jakobs D, Kaldenhoven N, Roza M, Hiddingh S, Meesters J, Voorhorst M, Gresnigt E, Wiegman L, Buijsse O, Andringa G, Overdijk MB, Doshi P, Sasser K, de Weers M, Parren PWHI (2014) Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087, SAR650984 and Ab79. Blood 124:3474. 10.1182/blood.V124.21.3474.3474
-
- Overdijk MB, Verploegen S, Bögels M, van Egmond M, Lammerts van Bueren JJ, Mutis T, Groen RW, Breij E, Martens AC, Bleeker WK, Parren PW (2015) Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. MAbs 7:311–321. 10.1080/19420862.2015.1007813 - PMC - PubMed
-
- Overdijk MB, Jansen JH, Nederend M, Lammerts van Bueren JJ, Groen RW, Parren PW, Leusen JH, Boross P (2016) The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcγ receptor–mediated cross-linking. J Immunol 197:807–813. 10.4049/jimmunol.1501351 - PubMed
-
- Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, Syed K, Liu K, van de Donk NWCJ, Weiss BM, Ahmadi T, Lokhorst HM, Mutis T, Sasser AK (2016) Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 128:384–394. 10.1182/blood-2015-12-687749 - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

