Extended fragrance ingredients surveillance study (EFISS)-protocol for a clinical surveillance study on contact allergy to 7 fragrance materials in widespread use but hitherto not systematically patch tested
- PMID: 40407895
- PMCID: PMC12102106
- DOI: 10.1007/s00403-025-04286-9
Extended fragrance ingredients surveillance study (EFISS)-protocol for a clinical surveillance study on contact allergy to 7 fragrance materials in widespread use but hitherto not systematically patch tested
Abstract
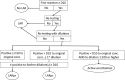
Contact allergy (CA) is not uncommon in the population, including to various fragrance allergens. If not diagnosed correctly, allergic contact dermatitis (ACD) may ensue, because targeted allergen avoidance is not possible. The primary objective of the study is to estimate the prevalence of CA to seven fragrance materials in patients with suspected ACD across Europe. Based on the outcome, a conclusion will be drawn as to whether present risk management regarding maximum recommended concentrations of each of these, based on quantitative risk assessment (QRA2), is adequate. The planned study is a surveillance study based on consecutive patients, patch tested in 10 European departments of dermatology with a series of allergens as indicated by their personal history, including the European baseline series, supplemented with the seven additional fragrance ingredients. The patch test procedure will follow the guideline of the European Society of Contact Dermatitis (ESCD) with additional standardization procedures. The envisaged sample size is 8100; recruitment will be in three data cycles with brief intervals allowing for descriptive interim analyses. Those patients reacting positively to any of the study allergens will be followed-up specifically to identify the source of sensitizing and/or eliciting exposure(s). Results will inform risk reassessment and subsequent risk management measures. Study results will be published in an open-access peer-reviewed scientific journal. Structured post-marketing surveillance of consumer risk of contact allergy by monitoring prevalences of positive patch test reactions in a dedicated European expert network is developed which can serve as a model for further chemicals. Important outcomes will be either a confirmation of effectiveness of risk management measures in place, or alternatively identifying aspects needing improvement (for certain cosmetic product categories). DRKS registration (DRKS00033263) 16.09.2024, mirrored at https://trialsearch.who.int.
Keywords: Contact allergy; Cross-sectional study; Epidemiological surveillance; Fragrance ingredients.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Wolfgang Uter receives research funds directed to the department by the International Fragrance Association (IFRA). Jim Bridges: is a member of the IDEA Supervisory Group; IDEA being sponsored by the International Fragrance Association. Margarida Gonçalo: Has participated in lectures and/or advisory boards and/or clinical trials for Abbvie, Almiral, Amgen, Astra-Zeneca, Biogen, Leo, Lilly, Novartis, Pfizer, Sanofi, Takeda. Stamatis Gregoriou: Lectures and/or advisory boards for Abbvie, Leo, Novartis, Pfizer, Sanofi, Lilly, UCB, Pierre Fabre, L’Oreal. Claudia C.V. Lang: is PI or investigator in several clinical trials, has received speaker fees, advisory fees and/or traveling reimbursement from the following companies: AbbVie, Amgen, ALK, Blueprint, Eli Lilly, Galderma, Incyte, Leo Pharma, Menarini AG, Novartis, Pfizer, Sanofi, Thermo Fisher. Suzana Ljubojevic Hadzavdic is a principal investigator of clinical studies for atopic dermatitis (Abbvie, Amgen) and for chronic spontaneous urticaria (Novartis). She wrote expert opinions and was lecturer for Lilly, Abbvie, Sanofi and Dr. Wolf. She is lecturer for Lilly, Novartis, Abbvie, Sanofi, Pliva, Bayer and Berlin-Chemie. Joseph Huggard works as a consultant for project IDEA sponsored by the International Fragrance Association (IFRA). Marléne Isaksson has worked as consultant for IDEA, sponsored by the International Fragrance Association (IFRA). Karl-Heinz Jöckel participates in the IDEA project sponsored by the International Fragrance Association (IFRA). Ian Kimber: is a member of the IDEA Supervisory Group; IDEA being sponsored by the International Fragrance Association. Elena Pezzolo has been a consultant, advisory board member, investigator, and/or speaker for Sanofi Genzyme, Pfizer, LEO Pharma, Eli Lilly, Almirall, Galderma, AbbVie, Novartis, Janssen, PellePharm, Boehringer Ingelheim, Bristol Myers Squibb. Thomas Rustemeyer has accepted honoraria for presentations from several pharmaceutical companies and is a member of the IDEA SG; IDEA being sponsored by the International Fragrance Association. Marie L.A. Schuttelaar has been a consultant, advisory board member, investigator, and/or speaker for Sanofi Genzyme, Regeneron Pharmaceuticals, Inc., Pfizer, LEO Pharma, Eli Lilly, Galderma, AbbVie, Novartis, Amgen. Cecilia Svedman participates in the IDEA project sponsored by the International Fragrance Association (IFRA). Matthias Vey is an employee of IFRA, International Fragrance Association. Magnus Bruze has lectured for Swedish Match and is a member of the Expert Panel for Fragrance Safety— http://fragrancesafetypanel.org/ . The other authors have no conflict of interest to declare. Ethical approval and consent to participate: Institutional Review Board of Friedrich-Alexander University Erlangen/Nürnberg granted approval on 2024-04-24 (24-103-br) concerning data analysis. The study is conducted in accordance with the Declaration of Helsinki. Ethical approval was sought from every institutional review board associated with the clinics involved. Informed consent to the standard diagnostic procedure (patch testing), to its study-related extension by 7 patch test preparations, and to the storage and transmission for analysis of pseudonymized or anonymized data (depending on the data capture system used) is sought from and documented for every included patient. Consent for publication: No identifiable patient characteristics will be published, i.e. not applicable.
Figures
References
-
- de Groot AC (2022) Patch Testing—Test Concentrations and Vehicles for 5200 Chemicals, 5th edn. Acdegroot Publishing, Netherlands
-
- SCCS (Scientific Committee on Consumer Safety) (2012) Opinion on fragrance allergens in cosmetic products, 26–27 June 2012 (SCCS/1459/11).
MeSH terms
Substances
Grants and funding
- EFISS/International Fragrance Association (IFRA)
- EFISS/International Fragrance Association (IFRA)
- EFISS/International Fragrance Association (IFRA)
- EFISS/International Fragrance Association (IFRA)
- EFISS/International Fragrance Association (IFRA)
- EFISS/International Fragrance Association (IFRA)
- EFISS/International Fragrance Association (IFRA)
- EFISS/International Fragrance Association (IFRA)
- EFISS/International Fragrance Association (IFRA)
- EFISS/International Fragrance Association (IFRA)
- EFISS/International Fragrance Association (IFRA)
- EFISS/International Fragrance Association (IFRA)
- EFISS/International Fragrance Association (IFRA)
- EFISS/International Fragrance Association (IFRA)
- EFISS/International Fragrance Association (IFRA)
- EFISS/International Fragrance Association (IFRA)
- EFISS/International Fragrance Association (IFRA)
LinkOut - more resources
Full Text Sources