Chemoproteomics unveils Sofalcone targeting ribosomal proteins to inhibit protein synthesis in Staphylococcus aureus
- PMID: 40408044
- PMCID: PMC12102032
- DOI: 10.1186/s43556-025-00269-4
Chemoproteomics unveils Sofalcone targeting ribosomal proteins to inhibit protein synthesis in Staphylococcus aureus
Abstract
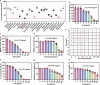
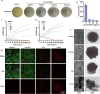
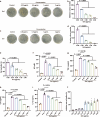
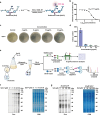
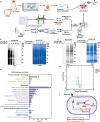
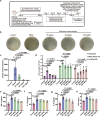
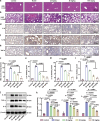
The escalating threat of antibiotic resistance, particularly in Staphylococcus aureus (including methicillin-resistant strains, MRSA), underscores the urgent need for novel therapeutics. Sofalcone (Sof), a chalcone derivative from Sophora subprostrata with established anti-inflammatory and anti-ulcer properties, exhibits promising yet underexplored antibacterial activity. Here, we demonstrate that Sof potently inhibits S. aureus and MRSA while showing minimal cytotoxicity in human cells. Notably, Sof synergized with amoxicillin, and significantly reduced the pathogenicity of S. aureus through inhibiting biofilm formation addressing key virulence factors. Through chemoproteomic profiling using a clickable Sof-derived probe, ribosomal proteins, specifically the 50S subunit protein rplB, were identified as primary targets. Sof covalently binds to rplB via cysteine residues, as validated by cellular thermal shift assays, microscale thermophoresis, and competition assays. Bio-orthogonal noncanonical amino acid tagging revealed that Sof disrupts bacterial protein synthesis by impairing ribosomal function, a mechanism distinct from conventional antibiotics. In a murine model of S. aureus-induced acute lung injury, Sof greatly reduced bacterial load in lungs, attenuated systemic inflammation, and mitigated histopathological damage. Its dual antibacterial and anti-inflammatory efficacy, coupled with activity against Gram-negative Escherichia coli, highlights broad-spectrum potential. This study unveils a covalent ribosomal-targeting strategy, positioning Sof as a multifaceted candidate against multidrug-resistant infections. Our findings bridge natural product pharmacology and mechanistic antimicrobial discovery, offering a template for combating the global antibiotic resistance crisis.
Keywords: Staphylococcus aureus; Antibiotics; Chemoproteomics; Drug-resistance; Ribosomal proteins; Sofalcone.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Animal experimentation and the corresponding protocol were approved by the Animal Ethics Committee of the Institute of Chinese Materia Medica China Academy of Chinese Medical Sciences (Ethical Number: 2023B299). Consent for publication: Not applicable. Competing interests: The authors declare no conflicts of interest to disclose.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
