Identification of Age- and Cataract-Related Changes in High-Density Lens Protein Aggregates
- PMID: 40408093
- PMCID: PMC12118509
- DOI: 10.1167/iovs.66.5.34
Identification of Age- and Cataract-Related Changes in High-Density Lens Protein Aggregates
Abstract
Purpose: Cataract is believed to be caused by protein-protein and protein-membrane aggregation in the eye lens. After middle age, there is extensive binding of crystallins to the lens cell membranes as evidenced by sedimentation at high densities. Multiple protein modifications have been linked with cataract, whereas others have been associated with aging. The purpose of this study was to characterize protein constituents within high density protein-membrane fractions from normal aged or cataractous lenses and to compare these proteins and their modifications.
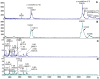
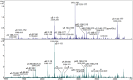
Methods: The inner nuclear regions of cataract or age-matched normal lenses were homogenized and proteins were separated using sucrose density gradient centrifugation. The low-density fractions (LDFs) and high-density fractions (HDFs) were analyzed by mass spectrometry using both top-down matrix-assisted desorption/ionization-mass spectrometry (MALDI-MS) and bottom-up liquid chromatography-tandem mass spectrometry (LC-MS/MS)-based proteomic methods. Quantification of low molecular weight crystallin peptides, deamidation, and isomerization were performed.
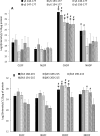
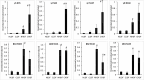
Results: Compared with normal aged-lenses, membrane-associated protein aggregates in high density fractions of cataract lenses exhibited significantly higher levels of γ-crystallins, as well as γS- and γD-crystallin C-terminal peptides. Deamidation of γ-crystallin, but not of β-crystallin, was increased in cataract lens membrane-bound aggregates. A very high level of Asp isomerization was detected in bound α-crystallins from both aged and cataract lenses.
Conclusions: Binding of crystallin aggregates to human lens cell membranes is associated with protein truncation, deamidation, and isomerization, and was observed in normal aged and cataract lenses. However, the protein aggregates bound to membranes in cataract lenses exhibit distinct modifications to γ-crystallins that may arise as a consequence of additional protein degradation.
Conflict of interest statement
Disclosure:
Figures

Similar articles
-
Crystallins in water soluble-high molecular weight protein fractions and water insoluble protein fractions in aging and cataractous human lenses.Mol Vis. 2004 Jul 19;10:476-89. Mol Vis. 2004. PMID: 15303090
-
Proteomic analysis of water insoluble proteins from normal and cataractous human lenses.Mol Vis. 2007 Sep 14;13:1680-94. Mol Vis. 2007. PMID: 17893670
-
Significance of interactions of low molecular weight crystallin fragments in lens aging and cataract formation.J Biol Chem. 2008 Mar 28;283(13):8477-85. doi: 10.1074/jbc.M705876200. Epub 2008 Jan 28. J Biol Chem. 2008. PMID: 18227073 Free PMC article.
-
Lens β-crystallins: the role of deamidation and related modifications in aging and cataract.Prog Biophys Mol Biol. 2014 Jul;115(1):21-31. doi: 10.1016/j.pbiomolbio.2014.02.004. Epub 2014 Mar 6. Prog Biophys Mol Biol. 2014. PMID: 24613629 Free PMC article. Review.
-
The Proteome of Cataract Markers: Focus on Crystallins.Adv Clin Chem. 2018;86:179-210. doi: 10.1016/bs.acc.2018.05.005. Epub 2018 Jul 13. Adv Clin Chem. 2018. PMID: 30144840 Review.
References
-
- Benedek GB. Cataract as a protein condensation disease: the Proctor Lecture. Invest Ophthalmol Vis Sci. 1997; 38(10): 1911–1921. - PubMed
-
- Jedziniak JA, Kinoshita JH, Yates EM, Hocker LO, Benedek GB.. On the presence and mechanism of formation of heavy molecular weight aggregates in human normal and cataractous lenses. Exp Eye Res. 1973; 15(2): 185–192. - PubMed
-
- Benedek GB. Theory of transparency of the eye. Appl Opt. 1971; 10(3): 459–473. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

