Regulatory network analysis of Dclk1 gene expression reveals a tuft cell-ILC2 axis that inhibits pancreatic tumor progression
- PMID: 40408246
- PMCID: PMC12270461
- DOI: 10.1016/j.celrep.2025.115734
Regulatory network analysis of Dclk1 gene expression reveals a tuft cell-ILC2 axis that inhibits pancreatic tumor progression
Abstract
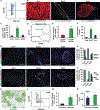
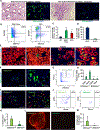
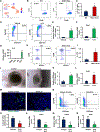
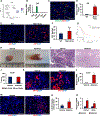
Doublecortin-like kinase 1 (Dclk1) expression identifies cells that are rare in normal pancreas but occur with an increased frequency in pancreatic neoplasia. The identity of these cells has been a matter of debate. We employed Dclk1 reporter mouse models and single-cell RNA sequencing (scRNA-seq) to define Dclk1-expressing cells. In normal pancreas, Dclk1 identifies subsets of ductal, islet, and acinar cells. In pancreatic neoplasia, Dclk1 identifies several cell populations, among which acinar-to-ductal metaplasia (ADM)-like cells and tuft-like cells are predominant. These two populations play opposing roles, with Dclk1+ ADM-like cells sustaining and Dclk1+ tuft-like cells restraining tumor progression. The generation of Dclk1+ tuft-like cells requires the transcription factor SPIB and is sustained by a paracrine loop involving type 2 innate lymphoid cells (ILC2s) and cancer-associated fibroblasts (CAFs) that provide interleukin (IL)-13 and IL-33, respectively. Dclk1+ tuft-like cells release angiotensinogen to restrain tumor progression. Overall, our study defines pancreatic Dclk1+ cells and unveils a protective tuft cell-ILC2 axis against pancreatic neoplasia.
Keywords: CP: Cancer; Dclk1; ILC2; pancreatic neoplasia; tuft cell.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests P.L. is director of Single-Cell Systems Biology at DarwinHealth, Inc., a company that has licensed some of the algorithms used in this article from Columbia University. A.C. is founder, equity holder, and consultant of DarwinHealth, Inc. Columbia University is also an equity holder in DarwinHealth, Inc.
Figures



Update of
-
Regulatory network analysis of Dclk1 gene expression reveals a tuft cell-ILC2 axis that inhibits pancreatic tumor progression.bioRxiv [Preprint]. 2024 Oct 12:2024.08.30.610508. doi: 10.1101/2024.08.30.610508. bioRxiv. 2024. Update in: Cell Rep. 2025 Jun 24;44(6):115734. doi: 10.1016/j.celrep.2025.115734. PMID: 39257805 Free PMC article. Updated. Preprint.
Similar articles
-
Regulatory network analysis of Dclk1 gene expression reveals a tuft cell-ILC2 axis that inhibits pancreatic tumor progression.bioRxiv [Preprint]. 2024 Oct 12:2024.08.30.610508. doi: 10.1101/2024.08.30.610508. bioRxiv. 2024. Update in: Cell Rep. 2025 Jun 24;44(6):115734. doi: 10.1016/j.celrep.2025.115734. PMID: 39257805 Free PMC article. Updated. Preprint.
-
FRA1 controls acinar cell plasticity during murine KrasG12D-induced pancreatic acinar to ductal metaplasia.Dev Cell. 2024 Nov 18;59(22):3025-3042.e7. doi: 10.1016/j.devcel.2024.07.021. Epub 2024 Aug 22. Dev Cell. 2024. PMID: 39178842
-
Tuft cells are required for a rhinovirus-induced asthma phenotype in immature mice.JCI Insight. 2024 Jan 23;9(2):e166136. doi: 10.1172/jci.insight.166136. JCI Insight. 2024. PMID: 38061015 Free PMC article.
-
[Prognostic value of molecular markers IDO1, DCLK1 and FOXC1 in Triple-negative breast cancer: morphological and clinical correlations].Arkh Patol. 2025;87(3):88-96. doi: 10.17116/patol20258703188. Arkh Patol. 2025. PMID: 40531562 Review. Russian.
-
Advances in tuft cells, a chemosensory cell in sequential diseases of the pancreas.Biochim Biophys Acta Rev Cancer. 2023 Jul;1878(4):188911. doi: 10.1016/j.bbcan.2023.188911. Epub 2023 May 12. Biochim Biophys Acta Rev Cancer. 2023. PMID: 37182665 Review.
References
-
- Brembeck FH, Schreiber FS, Deramaudt TB, Craig L, Rhoades B, Swain G, Grippo P, Stoffers DA, Silberg DG, and Rustgi AK (2003). The mutant K-ras oncogene causes pancreatic periductal lymphocytic infiltration and gastric mucous neck cell hyperplasia in transgenic mice. Cancer Res 63, 2005–2009. - PubMed
-
- Guerra C, Schuhmacher AJ, Cañamero M, Grippo PJ, Verdaguer L, Pérez-Gallego L, Dubus P, Sandgren EP, and Barbacid M (2007). Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 11, 291–302. 10.1016/j.ccr.2007.01.012. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

