Autophagy-mediated downregulation of AXL and TIM-1 promotes sustained Zika virus infection
- PMID: 40408405
- PMCID: PMC12130882
- DOI: 10.1073/pnas.2427241122
Autophagy-mediated downregulation of AXL and TIM-1 promotes sustained Zika virus infection
Abstract
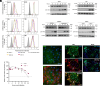
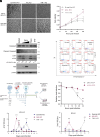
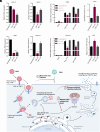
Zika virus (ZIKV) infection can lead to a variety of clinical outcomes, including severe congenital abnormalities. The phosphatidylserine receptors AXL and TIM-1 are recognized as critical entry factors for ZIKV in vitro. However, it remains unclear whether and how ZIKV regulates these receptors during infection. In this study, we investigated AXL and TIM-1 expression in human lung adenocarcinoma epithelial A549 cells, glioblastoma U87 cells, and embryonic stem cell-derived trophoblasts following ZIKV infection. We found that both the Asian strain FSS13025 and the African strain MR766 of ZIKV downregulate AXL, with a milder effect on TIM-1. We identified several ZIKV proteins, notably envelope (E), NS2A, NS3, and NS4B, that contribute to this downregulation. Notably, treatment with lysosomal inhibitor NH4Cl or the autophagy inhibitor 3-methyladenine mitigated the AXL/TIM-1 downregulation, indicating autophagy's involvement in the process. Importantly, this downregulation facilitates sustained viral replication and promotes viral spread by preventing superinfection and limiting cell death, which is also associated with impaired innate immune signaling. Our findings uncover a mechanism by which ZIKV downregulates entry factors to enhance prolonged viral replication and spread.
Keywords: AXL; TIM-1; ZIKV; autophagy; downregulation.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


Update of
-
Autophagy-Mediated Downregulation of AXL and TIM-1 Promotes Sustained Zika Virus Infection.bioRxiv [Preprint]. 2025 Jan 2:2024.12.31.630961. doi: 10.1101/2024.12.31.630961. bioRxiv. 2025. Update in: Proc Natl Acad Sci U S A. 2025 May 27;122(21):e2427241122. doi: 10.1073/pnas.2427241122. PMID: 39803534 Free PMC article. Updated. Preprint.
References
-
- Honein M. A., et al. , Birth defects among fetuses and infants of US women with evidence of possible Zika virus infection during pregnancy. JAMA 317, 59–68 (2017). - PubMed
-
- Santos T. d., et al. , Zika virus and the Guillain-Barré syndrome—Case series from seven countries. N. Engl. J. Med. 375, 1598–1601 (2016). - PubMed
-
- Pierson T. C., Diamond M. S., The emergence of Zika virus and its new clinical syndromes. Nature 560, 573–581 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

