Surgical techniques and outcome assessment of a novel vascularized orthotopic rodent whole eye transplantation model
- PMID: 40408444
- PMCID: PMC12101781
- DOI: 10.1371/journal.pone.0311392
Surgical techniques and outcome assessment of a novel vascularized orthotopic rodent whole eye transplantation model
Abstract
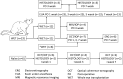
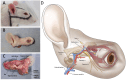
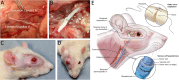
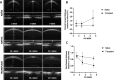
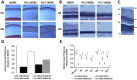
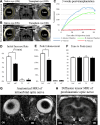
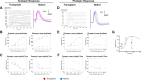
Currently there are no surgical solutions to restore vision in the irreversibly blind. Whole eye transplantation (WET), is an appealing surgical approach for restoration, replacement, and reconstruction of nonfunctioning eyes. Development of a reliable animal model to test the integrity and functionality of the transplanted eye is an essential step towards clinical whole eye transplantation. This study presents a feasible vascularized orthotopic eye transplantation preclinical rat model to study the structural and functional outcomes of whole eye transplantation. Syngeneic orthotopic transplants were performed in rats, involving anastomoses between carotid arteries, external jugular veins, and optic nerve coaptations of donors and recipients. The transplanted and recipient native eyes were assessed by ocular exam under anesthesia, optical coherence tomography (OCT), histology, magnetic resonance imaging and electroretinography. A 100% surgical survival rate of recipients with maintained long-term health demonstrated this to be a reliable and reproducible model. Assessment from clinical examination under anesthesia revealed that segments of native eyes appeared normal throughout the duration of the study, but transplanted eyes presented mild chemosis of the eye lids, mild ciliary flush of the conjunctiva, cornea neovascularization, mild engorgement of the vessels in the iris, and mild opacities in the lens in some animals. Most of these findings improved over time after transplantation. Doppler optical coherence tomography corroborated the presence of blood flow in transplanted retinas. There was no significant difference in measured IOP between native and transplanted eyes. Both histology and OCT scans demonstrated increased central corneal thickness and decreased total retinal thickness in transplanted eyes. Transplanted eyes exhibit minimal scotopic and photopic ERG responses. To date, no other vascularized orthotopic rodent WET transplantation models have been described in the literature. As functional visual return remains the ultimate goal, this model provides a foundation for future translational strategies and is ideal for testing immunomodulatory, neuroprotective, and neuroregenerative approaches either individually or in combination, as required for total human eye allotransplantation (THEA) to become a clinical reality.
Copyright: © 2025 Li et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- GBD 2019 Blindness and Vision Impairment Collaborators, Vision Loss Expert Group of the Global Burden of Disease Study. Trends in prevalence of blindness and distance and near vision impairment over 30 years: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9(2):e130–43. doi: 10.1016/S2214-109X(20)30425-3 - DOI - PMC - PubMed
-
- Bourne RR, Stevens GA, White RA, Smith JL, Flaxman SR, Price H, et al.. Causes of vision loss worldwide, 1990-2010: a systematic analysis. Lancet Glob Health. 2013;1(6):e339-49. - PubMed
-
- Stevens GA, White RA, Flaxman SR, Price H, Jonas JB, Keeffe J, et al.. Global prevalence of vision impairment and blindness: magnitude and temporal trends, 1990-2010. Ophthalmology. 2013;120(12):2377–84. - PubMed
-
- Kollar B, Tasigiorgos S, Dorante MI, Carty MJ, Talbot SG, Pomahac B. Innovations in reconstructive microsurgery: Reconstructive transplantation. J Surg Oncol. 2018;118(5):800–6. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

