Randomized Phase III Trial of Ramucirumab Beyond Progression Plus Irinotecan in Patients With Ramucirumab-Refractory Advanced Gastric Cancer: RINDBeRG Trial
- PMID: 40408613
- PMCID: PMC12199805
- DOI: 10.1200/JCO.24.01119
Randomized Phase III Trial of Ramucirumab Beyond Progression Plus Irinotecan in Patients With Ramucirumab-Refractory Advanced Gastric Cancer: RINDBeRG Trial
Abstract
Purpose: Continuous use of antiangiogenic agents has demonstrated survival benefits in various cancers. This trial aimed to compare the efficacy and safety of ramucirumab plus irinotecan with irinotecan monotherapy as a third- or later-line treatment for patients with advanced or recurrent gastric or gastroesophageal cancer (AGC) that has progressed on previous ramucirumab-based chemotherapy.
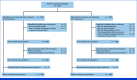
Methods: Patients age 20 years and older with AGC, who had experienced disease progression during ramucirumab-based chemotherapy, were randomly assigned to receive either ramucirumab plus irinotecan or irinotecan monotherapy. The primary end point was overall survival (OS) expecting a hazard ratio (HR) of 0.77 (a power of 80% and a significance level of one-sided 0.05). Secondary end points included progression-free survival (PFS), response rate, disease control rate (DCR), and safety.
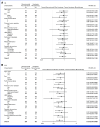
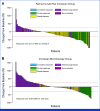
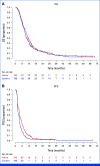
Results: Between February 2017 and August 2022, 402 patients in Japan were randomly assigned to receive ramucirumab plus irinotecan (n = 202) or irinotecan monotherapy (n = 200). The median OS was 9.4 months in the combination arm and 8.5 months in the monotherapy arm, with an adjusted HR of 0.91 (95% CI, 0.74 to 1.12; P = .49). PFS was improved (median, 3.8 v 2.8 months; HR, 0.72 [95% CI, 0.59 to 0.89]; P = .002), while the DCR was significantly better (64.4% v 52.1%; P = .03) with the combination therapy. The adverse events of the combination therapy were manageable.
Conclusion: Adding ramucirumab to irinotecan does not provide a significant advantage in OS over irinotecan alone in patients with AGC who have progressed during ramucirumab-containing chemotherapy.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures


References
-
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–263. - PubMed
-
- Hironaka S, Ueda S, Yasui H, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol. 2013;31:4438–4444. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

