Conformational Switch in the Alpha-Synuclein C-Terminal Domain Directs Its Fibril Polymorphs
- PMID: 40410120
- PMCID: PMC12223483
- DOI: 10.1002/chem.202500650
Conformational Switch in the Alpha-Synuclein C-Terminal Domain Directs Its Fibril Polymorphs
Abstract
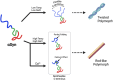
α-Synuclein (αSyn) inclusions are a pathological hallmark of several neurodegenerative disorders. While cryo-electron microscopy studies have revealed distinct fibril polymorphs across different synucleinopathies, the molecular switches controlling polymorphism remain unveiled. In this study, we found that fibril morphology is associated with the conformational state of monomeric αSyn. Through systematic evaluation of the ionic strength and temperature, we generated two distinct polymorphs: a twisted morphology at low ionic strength and temperature, and a rod-like morphology at higher ionic strength and temperature. Using solid-state NMR, we revealed that both polymorphs share a highly conserved core structure, with morphological differences arising probably from distinct structural arrangements at the protofilament interfaces. Furthermore, we found that a specific conformational change in the C-terminal domain of the monomeric αSyn serves as a molecular switch for the formation of polymorphs. Interestingly, this conformational change can also be triggered by calcium binding to the C-terminus, connecting environmental factors to specific fibril architectures. Our results reveal a conformational role for the C-terminal domain that influences αSyn fibril morphology, providing significant insights into the fibrogenesis of αSyn.
Keywords: NMR; alpha synuclein; amyloid fibrils; fibril polymorphism; protein folding; proteins.
© 2025 The Author(s). Chemistry – A European Journal published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
- JP24H00045/JSPS KAKENHI
- JP22K07516/JSPS KAKENHI
- JP22H02951/JSPS KAKENHI
- JP22K15643/JSPS KAKENHI
- JP23K24212/JSPS KAKENHI
- JP23K18255/JSPS KAKENHI
- JPJSCCA20180007/JSPS Core-to-Core Program A Advanced Research Networks
- JPMJFR231L/JST FOREST Program
- JP24ek0109771h/Japan Agency for Medical Research and Development
- JP24gm1910008/Japan Agency for Medical Research and Development
- JP22gm1410014/Japan Agency for Medical Research and Development
LinkOut - more resources
Full Text Sources

