PfPPM2 signalling regulates asexual division and sexual conversion of human malaria parasite Plasmodium falciparum
- PMID: 40410154
- PMCID: PMC12102381
- DOI: 10.1038/s41467-025-59476-w
PfPPM2 signalling regulates asexual division and sexual conversion of human malaria parasite Plasmodium falciparum
Abstract
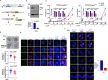
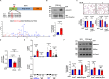
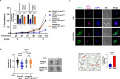
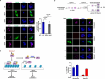
Malaria parasite undergoes interesting developmental transition in human and mosquito host. While it divides asynchronously in the erythrocytes, it switches to sexual forms, which is critical for disease transmission. We report a novel signalling pathway involving Protein Phosphatase PfPPM2, which regulates asexual division of Plasmodium falciparum as well as its conversion to sexual forms. PfPPM2 may regulate the phosphorylation of key proteins involved in chromatin remodelling and protein translation. One of the key PfPPM2-targets was Heterochromatin Protein 1 (HP1), a regulator of heritable gene silencing which contributes to both mitotic proliferation as well as sexual commitment of the parasite. PfPPM2 promotes sexual conversion by regulating the interaction between HP1, H3K9me3 and chromatin and it achieves this by dephosphorylating S33 of HP1. PfPPM2 also regulates protein synthesis in the parasite by repressing the phosphorylation of initiation factor eIF2α, which is likely to contribute to parasite division and possibly sexual differentiation.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- World Malaria Report. Geneva: World Health Organization; 2022. Licence: CC BY-NC-SA 3.0 IGO. (2022).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

