Fusogenic lipid nanoparticles for rapid delivery of large therapeutic molecules to exosomes
- PMID: 40410169
- PMCID: PMC12102247
- DOI: 10.1038/s41467-025-59489-5
Fusogenic lipid nanoparticles for rapid delivery of large therapeutic molecules to exosomes
Abstract
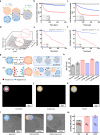
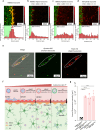
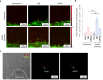
Exosomes, as cell-derived lipid nanoparticles, are promising drug carriers because they can traverse challenging physiological barriers such as the blood-brain barrier (BBB). However, a major obstacle in utilizing exosomes as drug carriers is loading large therapeutic molecules without compromising the structural integrity of embedded biomolecules. Here, we introduce a membrane fusion method utilizing fusogenic lipid nanoparticles, cubosomes, to load large molecules into exosomes in a non-destructive manner. When the drug-loaded cubosome and exosome solutions are simply mixed, membrane fusion is completed in just 10 min. Our method effectively loads doxorubicin and immunoglobulin G into exosomes. Moreover, even the most challenging molecule-mRNA-is loaded with nearly 100% efficiency, demonstrating the versatility of our approach. In terms of biological behavior, the resulting hybrid exosomes preserve the functional behavior of exosomes in BBB uptake and penetration. Surprisingly, controlling exosome-to-cubosome ratios allows precise control over BBB uptake and transport. Furthermore, these hybrid exosomes retain cell-specific delivery properties, preserving the targeted delivery functions dictated by their exosomal origin. This study demonstrates the feasibility of a mix-and-load method for rapid and efficient drug loading into exosomes, with significant potential for the treatment of neurological diseases.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The Korea Institute of Science and Technology is in the process of applying for a Korean invention patent (10-2025-0058245) related to the subject matter of this paper.
Figures




References
MeSH terms
Substances
Grants and funding
- RS-2024-00396818/Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (iPET)
- RS-2024-00396818/Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (iPET)
- RS-2024-00396818/Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (iPET)
- RS-2023-00209955/National Research Foundation of Korea (NRF)
- RS-2023-00209955/National Research Foundation of Korea (NRF)
- RS-2024-00424551/National Research Foundation of Korea (NRF)
- RS-2024-00395393/National Research Foundation of Korea (NRF)
- RS-2023-00209955/National Research Foundation of Korea (NRF)
- RS-2024-00424551/National Research Foundation of Korea (NRF)
- RS-2024-00395393/National Research Foundation of Korea (NRF)
LinkOut - more resources
Full Text Sources

