Metabolomics biomarkers of frailty: a longitudinal study of aging female and male mice
- PMID: 40410187
- PMCID: PMC12102153
- DOI: 10.1038/s41514-025-00237-w
Metabolomics biomarkers of frailty: a longitudinal study of aging female and male mice
Abstract
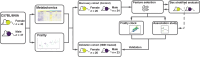
Frailty is an age-related geriatric syndrome. We performed a longitudinal study of aging female (n = 40) and male (n = 47) C57BL/6NIA mice, measured frailty index and derived metabolomics data from plasma. We identify age-related differentially abundant metabolites, determine frailty-related metabolites, and generate frailty features, both in the whole cohort and sex-stratified subgroups. Using the features, we perform an association study and build a metabolomics-based frailty clock. We find that frailty-related metabolites are enriched for amino acid metabolism and metabolism of cofactors and vitamins, include ergothioneine, tryptophan and alpha-ketoglutarate, and present sex dimorphism. We identify B vitamin metabolism related flavin-adenine dinucleotide and pyridoxate as female-specific frailty biomarkers, and lipid metabolism related sphingomyelins, glycerophosphoethanolamine and glycerophosphocholine as male-specific frailty biomarkers. These associations are confirmed in a validation cohort, with ergothioneine and perfluorooctanesulfonate identified as robust frailty biomarkers. Our results identify sex-specific metabolite frailty biomarkers, and shed light on potential mechanisms.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: D.A.S. is a founder, equity owner, advisor to, director of, board member of, consultant to, investor in and/or inventor on patents licensed to Revere Biosensors, UpRNA, GlaxoSmithKline, Wellomics, DaVinci Logic, InsideTracker (Segterra), Caudalie, Animal Biosciences, Longwood Fund, Catalio Capital Management, Frontier Acquisition Corporation, AFAR (American Federation for Aging Research), Life Extension Advocacy Foundation (LEAF), Cohbar, Galilei, EMD Millipore, Zymo Research, Immetas, Bayer Crop Science, EdenRoc Sciences (and affiliates Arc-Bio, Dovetail Genomics, Claret Bioscience, MetroBiotech, Astrea, Liberty Biosecurity and Delavie), Life Biosciences, Alterity, ATAI Life Sciences, Levels Health, Tally (aka Longevity Sciences) and Bold Capital. D.A.S. is an inventor on a patent application filed by Mayo Clinic and Harvard Medical School that has been licensed to Elysium Health. Additional info on D.A.S. affiliations can be found at https://sinclair.hms.harvard.edu/david-sinclairs-affiliations . The other authors declare no competing interests.
Figures






Update of
-
Metabolomics biomarkers of frailty: a longitudinal study of aging female and male mice.bioRxiv [Preprint]. 2025 Jan 22:2025.01.22.634160. doi: 10.1101/2025.01.22.634160. bioRxiv. 2025. Update in: NPJ Aging. 2025 May 23;11(1):40. doi: 10.1038/s41514-025-00237-w. PMID: 39896479 Free PMC article. Updated. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources

