Perfusion-based ex vivo culture of frozen ovarian cancer tissues with preserved tumor microenvironment
- PMID: 40410344
- PMCID: PMC12102267
- DOI: 10.1038/s41698-025-00941-6
Perfusion-based ex vivo culture of frozen ovarian cancer tissues with preserved tumor microenvironment
Abstract
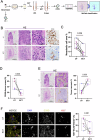
Ovarian cancer (OC) poses significant treatment challenges due to late-stage diagnosis and a complex tumor microenvironment contributing to therapy resistance. We optimized a U-CUP perfusion-based bioreactor method to culture patient-derived primary and metastatic OC specimens, demonstrating that perfusion better preserves cancer cell viability and proliferation, both when fresh and slow-frozen tissues were used. Perfused cultures maintained key microenvironment components, including cancer-associated fibroblasts, endothelial and immune cells. Genetic analysis confirmed the retention in culture of tumor-specific driver mutations. We hence challenged ad hoc generated cisplatin-sensitive and resistant OC cells with cisplatin during growth in U-CUP, validating our system for the testing of drug response. Finally, treatment of slow-frozen OC tissues with carboplatin/paclitaxel revealed different degrees of response to treatment, as indicated by variations in tumor necrosis and number of residual PAX8+ cells, providing the bases for the prompt evaluation of OC standard chemotherapy efficacy in our ex vivo system.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Ivan Martin is Cellec Biotek AG founder, shareholder, and member of the board. Manuele Giuseppe Muraro is Cellec Biotek AG scientific advisor and the inventor of the patent WO2015181185A1. All the other authors declare no competing interests.
Figures
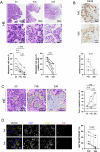
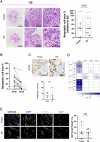
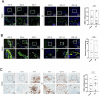
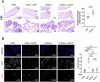
References
-
- Cancer of the Ovary - Cancer Stat Facts (2025). SEER. https://seer.cancer.gov/statfacts/html/ovary.html.
-
- Arora, T., Mullangi, S., Vadakekut, E. S. & Lekkala, M. R. Epithelial Ovarian Cancer. In StatPearls (StatPearls Publishing) (2024). - PubMed
Grants and funding
- 24494/Associazione Italiana Ricerca sul Cancro (AIRC) project IG 2020
- 171037/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung
- 171037/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung
- 171037/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung
- 171037/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung
LinkOut - more resources
Full Text Sources

