On the ultra-rapid mixing in two colliding Leidenfrost drops
- PMID: 40410384
- PMCID: PMC12102151
- DOI: 10.1038/s41598-025-02940-w
On the ultra-rapid mixing in two colliding Leidenfrost drops
Abstract
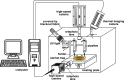
Leidenfrost drop can accelerate chemical reactions, offering great potential for droplet-based chemical reactors. By leveraging their motion on heated surfaces with micro-rachets, we demonstrate that mixing can be further enhanced through head-on collisions of two Leidenfrost drops. This study identifies three mixing stages. In Stage I, collision dynamics and film drainage between drops control coalescence, with surface tension disparities prolonging Stage I for heterogeneous drops. Stage II is driven by advective transport, though viscous effect from deformation can slow internal flow. In Stage III, oscillations promote mixing. For identical drops, complete mixing can be achieved within 2-3 oscillations. However, when drops with different boiling points collide, bubble nucleation emerges from the contact surface. Boiling not only prolongs the transition to Stage III or even disrupts stable oscillations but also hinders mixing through selective evaporation. In this study, the most rapid mixing occurs when two 10 µl Leidenfrost drops of water coalesce. Complete mixing is achieved within 100 ms, about two orders of magnitude faster than conventional methods. The results provide insights into optimizing Leidenfrost drop reactors and highlight the benefits of the extreme mobility of the Leidenfrost state for applications requiring rapid mixing.
Keywords: Binary collision; Coalescence; Leidenfrost drop; Mixing process; Surface oscillation; Unequal size.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures







Similar articles
-
Triple Leidenfrost Effect: Preventing Coalescence of Drops on a Hot Plate.Phys Rev Lett. 2021 Nov 12;127(20):204501. doi: 10.1103/PhysRevLett.127.204501. Phys Rev Lett. 2021. PMID: 34860033
-
Leidenfrost Effect-Induced Chaotic Vortex Flow for Efficient Mixing of Highly Viscous Droplets.Adv Mater. 2024 Oct;36(40):e2409192. doi: 10.1002/adma.202409192. Epub 2024 Aug 27. Adv Mater. 2024. PMID: 39188204
-
On explosive boiling of a multicomponent Leidenfrost drop.Proc Natl Acad Sci U S A. 2021 Jan 12;118(2):e2016107118. doi: 10.1073/pnas.2016107118. Proc Natl Acad Sci U S A. 2021. PMID: 33419924 Free PMC article.
-
A Review on the Coalescence of Confined Drops with a Focus on Scaling Laws for the Growth of the Liquid Bridge.Micromachines (Basel). 2023 Oct 31;14(11):2046. doi: 10.3390/mi14112046. Micromachines (Basel). 2023. PMID: 38004903 Free PMC article. Review.
-
Effect of surface topography and wettability on the Leidenfrost effect.Nanoscale. 2017 May 18;9(19):6219-6236. doi: 10.1039/c7nr01845b. Nanoscale. 2017. PMID: 28470271 Review.
References
-
- Taniguchi, T., Torii, T. & Higuchi, T. Chemical reactions in microdroplets by electrostatic manipulation of droplets in liquid media. Lab. Chip2(1), 19–23. 10.1039/B108739H (2002). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

