Patient-derived esophageal adenocarcinoma organ chip: a physiologically relevant platform for functional precision oncology
- PMID: 40410759
- PMCID: PMC12102830
- DOI: 10.1186/s12967-025-06593-1
Patient-derived esophageal adenocarcinoma organ chip: a physiologically relevant platform for functional precision oncology
Abstract
Background: Esophageal adenocarcinoma (EAC) is the sixth most deadly cancer worldwide, with increasing incidence in North America. As no targeted therapy or immunotherapy has revolutionized the management of EAC, chemotherapy is the only standard of care. Most patients with EAC experience poor outcomes because of the inherent or acquired resistance to chemotherapy.
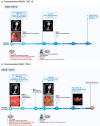
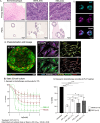
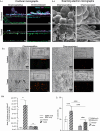
Methods: Adapting a patient-centered approach, we leveraged a microfluidic cell culture technology platform (Emulate), organoids derived from treatment-naive patient tumors or adjacent normal tissues, and patient-matched cancer-associated or normal fibroblasts respectively, to develop a novel, physiologically relevant, high-fidelity preclinical esophagus-on-a-chip model. H&E, immunofluorescence staining, live/dead assay, LDH assay, and ELISA-based detection of tumor biomarkers were used to assess treatment responses.
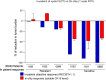
Results: Each patient-specific stroma-inclusive microfluidic esophageal adenocarcinoma on-a-chip (EAC chip) faithfully recreates the tumor-stroma interface while preserving the full diversity of two cell types (epithelia and fibroblasts), genetic landscapes and histological architecture of the source tumors. EAC chips also accurately predict the response to neoadjuvant chemotherapy (NACT) within a clinically useful timeframe (approx. 12 days). A docetaxel-based triplet chemotherapy regimen matched with the treatment of the source patient was successfully perfused through the interstitial space within this model. Therefore, EAC chips more accurately recapitulate inpatient pathological and objective responses than the corresponding static 3D-organoid-only cultures.
Conclusions: Overall, this model is an effective tool for predicting patients' responses to chemotherapy and testing tumor- or stroma-targeted alternative therapies. Moreover, these high-fidelity, low-throughput EAC chips effectively complement high-throughput PDO culture-based drug testing and provide improved insights into drug efficacy before human studies.
Keywords: Cancer-associated fibroblasts; Esophageal adenocarcinoma; Organ-on-a-chip; Organoids.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Patient data were used in this study, with informed consent obtained from each patient prior to the diagnostic biopsy. The study was approved by the Research Ethics Board of the McGill University Health Centre (protocols 2007 − 856 and 2021–7681). Consent for publication: The study includes data from individuals who provided consent for their information without identity to be used exclusively for research purposes. Conflict of interest: Dr. Donald E. Ingber is a founder, board member, and chairs the SAB of Emulate Inc., and holds equity. The remaining authors disclose no conflicts.
Figures


References
-
- Morgan E, Soerjomataram I, Rumgay H, Coleman HG, Thrift AP, Vignat J, Laversanne M, Ferlay J, Arnold M. The global landscape of esophageal squamous cell carcinoma and esophageal adenocarcinoma incidence and mortality in 2020 and projections to 2040: new estimates from GLOBOCAN 2020. Gastroenterology. 2022;163(3):649–58. 10.1053/j.gastro.2022.05.054. - PubMed
-
- Ajani JA, D’Amico TA, Bentrem DJ, Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Farjah F, Gerdes H, et al. Esophageal and esophagogastric junction cancers, version 2.2023, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2023;21(4):393–422. 10.6004/jnccn.2023.0019. - PubMed
-
- Derouet MF, Allen J, Wilson GW, Ng C, Radulovich N, Kalimuthu S, Tsao MS, Darling GE, Yeung JC. Towards personalized induction therapy for esophageal adenocarcinoma: organoids derived from endoscopic biopsy recapitulate the pre-treatment tumor. Sci Rep. 2020;10(1):14514. 10.1038/s41598-020-71589-4. - PMC - PubMed
-
- Karakasheva TA, Gabre JT, Sachdeva UM, Cruz-Acuna R, Lin EW, DeMarshall M, Falk GW, Ginsberg GG, Yang Z, Kim MM, et al. Patient-derived organoids as a platform for modeling a patient’s response to chemoradiotherapy in esophageal cancer. Sci Rep. 2021;11(1):21304. 10.1038/s41598-021-00706-8. - PMC - PubMed
-
- Liu H, Wang X. Esophageal organoids: applications and future prospects. J Mol Med (Berl). 2023;101(8):931–45. 10.1007/s00109-023-02340-5. - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

