Injectable platelet-rich fibrin with vitamin C as an adjunct to non-surgical periodontal therapy in the treatment of stage-II periodontitis: a randomized controlled clinical trial
- PMID: 40410805
- PMCID: PMC12100897
- DOI: 10.1186/s12903-025-06115-x
Injectable platelet-rich fibrin with vitamin C as an adjunct to non-surgical periodontal therapy in the treatment of stage-II periodontitis: a randomized controlled clinical trial
Abstract
Background: Injectable platelet-rich fibrin (I-PRF) is an autologous fibrin matrix rich in leucocytes, platelets and growth factors, and could serve as a sustained-release vehicle for a variety of active biomolecules. The aim of the current randomized controlled trial was to compare the effect of vitamin C (VitC) with I-PRF as a locally delivered adjunct to professional mechanical plaque removal (PMPR) versus PMPR with local delivery of I-PRF or PMPR alone on non-surgical periodontal treatment (NSPT) outcomes of stage-II periodontitis.
Methodology: Forty-five patients (n = 45) diagnosed with stage-II grade A periodontitis were randomly assigned into test (PMPR + I-PRF/VitC; n = 15) or control groups (PMPR + I-PRF; n = 15 and PMPR; n = 15). Bleeding on probing (BOP; primary outcome), probing depth (PD), clinical attachment level (CAL), gingival margin (GM), plaque index (PI) and radiographic bone gain/loss (horizontal, vertical and total) were assessed at baseline, three- and six-months post-treatment. Post-operative pain was further assessed at second- and third-day post-treatment.
Results: Although BOP scores were lower in the PMPR + I-PRF/VitC group, the regression analysis revealed that gender was the only significant predictor for BOP, with females showing a reduced propensity (p < 0.05). Clinical and radiographic parameters significantly improved in all groups independently (p < 0.05). PD-reduction was 1.73 ± 0.59 mm, 1.67 ± 0.49 mm and 1.73 ± 0.59 mm, CAL-change was 1.33 ± 0.49 mm, 1.20 ± 0.56 mm and 0.93 ± 0.59 mm and GM-change was 0.40 ± 0.51 mm, 0.33 ± 0.49 mm and 0.73 ± 0.70 mm in the PMPR + I-PRF/VitC, PMPR + I-PRF and PMPR groups respectively. No intergroup differences were notable regarding BOP or changes in PD, CAL, GM, PI and radiographic bone measurements at three or six months relative to baseline (p > 0.05). Significantly lower pain scores at two and three days were notable in the PMPR + I-PRF/VitC and PMPR + I-PRF groups compared to the PMPR group (p < 0.05).
Conclusions: Apart from a positive effect on the patients' post operative pain perception, I-PRF with or without the addition of vitamin C does not additionally improve the clinical outcomes of PMPR alone in the NSPT of stage-II grade A periodontitis patients.
Trial registration: Trial registration. The study was retrospectively registered in the US National Institutes of Health Clinical Trials Registry (NCT05129267) on 2021-11-10.
Keywords: Ascorbic acid; Gingival pocket; Injections; Periodontitis; Plaque; Platelet-rich fibrin.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval and consent to participate: The study protocols involving human participants adhered to the ethical standards set by the institutional and/or national research committee and were conducted in accordance with the principles outlined in the 2013 Helsinki Declaration and its subsequent revisions, or similar ethical standards. Both the research protocol and informed consent form were approved by the Ethics Committee of the Faculty of Dentistry, Cairo University, Egypt in July 2021 (IRB: 13|7|21). All participants included in the study gave written informed consents before their participation. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
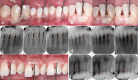

References
-
- Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and Peri-Implant diseases and conditions. J Clin Periodontol. 2018;45:S162–70. - PubMed
-
- Helal O, Gostemeyer G, Krois J, Fawzy El Sayed K, Graetz C, Schwendicke F. Predictors for tooth loss in periodontitis patients: systematic review and meta-analysis. J Clin Periodontol. 2019;46(7):699–712. - PubMed
-
- Graetz C, Dorfer CE, Kahl M, Kocher T, Fawzy El-Sayed K, Wiebe JF, et al. Retention of questionable and hopeless teeth in compliant patients treated for aggressive periodontitis. J Clin Periodontol. 2011;38(8):707–14. - PubMed
-
- Tomasi C, Abrahamsson KH, Apatzidou D. Subgingival instrumentation. Periodontol 2000. 2023. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

