Ubiquitination in hepatocellular carcinoma immunity
- PMID: 40410880
- PMCID: PMC12102898
- DOI: 10.1186/s12967-025-06592-2
Ubiquitination in hepatocellular carcinoma immunity
Abstract
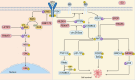
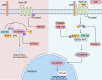
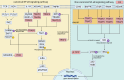
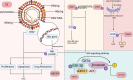
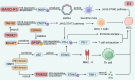
Hepatocellular carcinoma (HCC) is the sixth most prevalent malignancy worldwide, and represents a major global health challenge. While surgical resection at early stages offers favorable prognosis with 5-year survival rates exceeding 70%, the clinical reality in China reveals a contrasting scenario, where over 60% of patients present with advanced disease, resulting in a dramatic decline in 5-year survival to below 12.5%. The immunological landscape plays a pivotal role in HCC pathogenesis and progression, comprising two complementary arms: the innate immune system's rapid-response mechanism for immediate tumor surveillance and the adaptive immune system's antigen-specific targeting with immunological memory capabilities. Emerging evidence has highlighted ubiquitination, a sophisticated post-translational modification system, as a critical regulator of immune homeostasis in HCC pathogenesis. This molecular process exerts precise control through three primary mechanisms: (1) Modulation of immune cell activation thresholds via proteasomal degradation of signaling proteins, (2) Orchestrating immune cell differentiation through stability regulation of transcriptional factors, and (3) Maintenance of immune tolerance by dynamic modification of checkpoint regulators. Such multifaceted regulation affects both innate immune recognition pathways (e.g., NF-κB and STING signaling) and adaptive immune effectors (particularly T cell receptor signaling cascades). This comprehensive review establishes a threefold Objective: First, to elucidate the mechanistic interplay between ubiquitination networks and HCC-related immune dysregulation; Second, to systematically analyze how innate immune-associated ubiquitination events drive hepatocarcinogenesis through chronic inflammation modulation; and third, to critically evaluate recent clinical advances combining ubiquitination-targeted therapies (e.g., proteasome inhibitors and E3 ligase modulators) with immunotherapeutic regimens. Our synthesis revealed that strategic manipulation of ubiquitination pathways can potentiate PD-1/PD-L1 blockade efficacy while mitigating therapeutic resistance, particularly through modulation of tumor-associated macrophages and exhausted T cell populations. By integrating fundamental mechanistic insights with translational clinical data, this review provides a conceptual framework for the development of next-generation diagnostic biomarkers and rational therapeutic combinations. The proposed strategy of ubiquitination-immune axis modulation holds significant potential to transform current HCC management paradigms, offering new avenues for precision immunotherapy for this challenging malignancy.
Keywords: Adaptive immune; E3; HBV; HCC; Innate immune; Ubiquitination.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare that there is no conflict of interests.
Figures
Similar articles
-
From bench to bed: the tumor immune microenvironment and current immunotherapeutic strategies for hepatocellular carcinoma.J Exp Clin Cancer Res. 2019 Sep 9;38(1):396. doi: 10.1186/s13046-019-1396-4. J Exp Clin Cancer Res. 2019. PMID: 31500650 Free PMC article. Review.
-
Immunobiology and immunotherapy of HCC: spotlight on innate and innate-like immune cells.Cell Mol Immunol. 2021 Jan;18(1):112-127. doi: 10.1038/s41423-020-00572-w. Epub 2020 Nov 24. Cell Mol Immunol. 2021. PMID: 33235387 Free PMC article. Review.
-
Inhibition of KIF20A enhances the immunotherapeutic effect of hepatocellular carcinoma by enhancing c-Myc ubiquitination.Cancer Lett. 2024 Aug 28;598:217105. doi: 10.1016/j.canlet.2024.217105. Epub 2024 Jul 4. Cancer Lett. 2024. PMID: 38971490
-
Role and therapeutic potential of E3s in the tumor microenvironment of hepatocellular carcinoma.Front Immunol. 2024 Oct 31;15:1483721. doi: 10.3389/fimmu.2024.1483721. eCollection 2024. Front Immunol. 2024. PMID: 39544935 Free PMC article. Review.
-
Glycolytic activation of peritumoral monocytes fosters immune privilege via the PFKFB3-PD-L1 axis in human hepatocellular carcinoma.J Hepatol. 2019 Aug;71(2):333-343. doi: 10.1016/j.jhep.2019.04.007. Epub 2019 May 7. J Hepatol. 2019. PMID: 31071366
References
-
- Sangiovanni A, et al. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006;43:1303–10. - PubMed
-
- Ioannou GN, et al. Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2007;5:938–e945934. - PubMed
-
- Maucort-Boulch D, de Martel C, Franceschi S, Plummer M. Fraction and incidence of liver cancer attributable to hepatitis B and C viruses worldwide. Int J Cancer. 2018;142:2471–7. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

