Matrix stiffness enhances viability, migration, invasion and invadopodia formation of oral cancer cells via PI3K/AKT pathway in vitro
- PMID: 40410903
- PMCID: PMC12103043
- DOI: 10.1186/s40001-025-02666-5
Matrix stiffness enhances viability, migration, invasion and invadopodia formation of oral cancer cells via PI3K/AKT pathway in vitro
Abstract
Background: Oral cancer (OC) is one of the major types of cancer and the most common cause of cancer-related mortality in Asia. In recent years, matrix stiffness in the tumor microenvironment has been found to play an important role in regulating tumor cell behavior. However, the regulatory mechanisms associated with matrix stiffness in OC cells remain unclear.
Methods: In this study, polyacrylamide gels with different stiffness were prepared to simulate low versus high matrix stiffness environments in tumor tissues by adjusting the acrylamide and cross-linker concentrations. Subsequently, the effects of different stiffness on OC cell survival, migration, invasion and invadopodia formation were explored based on cell counting kit-8 (CCK-8), Transwell and confocal microscopy. Meanwhile, the levels of markers relevant to phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT), apoptosis (BAX and BCL2) as well as metastasis (Cadherin-1, CDH1; Cadherin-2, and CDH2) were calculated via western blotting and real-time quantitative PCR.
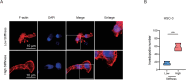
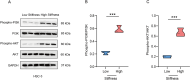
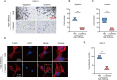
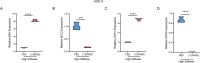
Results: According to the results, high matrix stiffness was seen to contribute to the increased number of migrated and invaded cells as well as the enhanced viability of OC cells, along with the aggravated invadopodia formation and the up-regulation in CDH2 and BCL2 levels yet the down-regulation in CDH1 and BAX levels. Elevated PI3K/AKT phosphorylation levels were also seen in high matrix stiffness-mediated OC cells, and the intervention using LY294002 could visibly overturned the effects of high matrix stiffness on the cell migration, invasion and invadopodia formation of OC cells.
Conclusions: This study reveals that matrix stiffness may enhance the invasiveness and anti-apoptotic ability of OC cells by activating the PI3K/AKT pathway, which provides a new idea for exploring the microenvironmental regulation of tumor mechanics and targeted intervention strategies.
Keywords: Invadopodia; Matrix stiffness; Metastasis; Oral cancer; PI3K/AKT pathway.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


Similar articles
-
S100A8 Mediates Autophagy and Apoptosis in Ovarian Cancer Cells via the PI3K/Akt Pathway.Discov Med. 2025 Jun;37(197):1117-1129. doi: 10.24976/Discov.Med.202537197.99. Discov Med. 2025. PMID: 40485527
-
[Kuwanon G inhibits growth, migration and invasion of gastric cancer cells by regulating the PI3K/AKT/mTOR pathway].Nan Fang Yi Ke Da Xue Xue Bao. 2024 Aug 20;44(8):1476-1484. doi: 10.12122/j.issn.1673-4254.2024.08.06. Nan Fang Yi Ke Da Xue Xue Bao. 2024. PMID: 39276043 Free PMC article. Chinese.
-
DMBT1 suppresses cell proliferation, migration and invasion in ovarian cancer and enhances sensitivity to cisplatin through galectin-3/PI3k/Akt pathway.Cell Biochem Funct. 2020 Aug;38(6):801-809. doi: 10.1002/cbf.3549. Epub 2020 May 19. Cell Biochem Funct. 2020. PMID: 32424818
-
Significance of kinase activity in the dynamic invadosome.Eur J Cell Biol. 2016 Nov;95(11):483-492. doi: 10.1016/j.ejcb.2016.07.002. Epub 2016 Jul 19. Eur J Cell Biol. 2016. PMID: 27465307 Review.
-
The involvement of mutant Rac1 in the formation of invadopodia in cultured melanoma cells.Exp Cell Res. 2016 Apr 10;343(1):82-88. doi: 10.1016/j.yexcr.2016.02.003. Epub 2016 Feb 10. Exp Cell Res. 2016. PMID: 26873115 Free PMC article. Review.
References
-
- Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. - PubMed
-
- Conway DI, Purkayastha M, Chestnutt IG. The changing epidemiology of oral cancer: definitions, trends, and risk factors. Br Dent J. 2018;225(9):867–73. - PubMed
-
- Li Q, et al. A review on salivary proteomics for oral cancer screening. Curr Issues Mol Biol. 2020;37:47–56. - PubMed
-
- Lan Y-Y, et al. Paclitaxel induces human KOSC3 oral cancer cell apoptosis through caspase pathways. Biocell. 2024;48(7):1047–54.
-
- Taneja N, et al. Understanding nanotechnology in the treatment of oral cancer: a comprehensive review. Crit Rev Ther Drug Carrier Syst. 2021;38(6):1–48. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

