The performance of plasma p-tau217 in Black middle-aged and older adults
- PMID: 40410928
- PMCID: PMC12101965
- DOI: 10.1002/alz.70288
The performance of plasma p-tau217 in Black middle-aged and older adults
Abstract
Introduction: Medical conditions prevalent in Black adults within the United States have been associated with plasma tau phosphorylated at threonine 217 (p-tau217); however, insufficient p-tau217 research has been conducted with Black adults.
Methods: Participants included n = 233 predominantly cognitively unimpaired adults enrolled in the African Americans Fighting Alzheimer's in Midlife study. Subsamples had creatinine (n = 137) and positron emission tomography (PET; amyloid-PET = 65 [amyloid-PET-positive = 16/65]; tau-PET = 70). We tested whether p-tau217 (ALZPath, Inc.) varied by medical condition and amyloid- and tau-PET-positivity status and assessed the diagnostic accuracy of p-tau217.
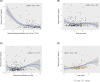
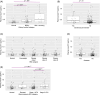
Results: Estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2, cardiovascular disease (CVD), and amyloid- and tau-PET-positive status demonstrated higher p-tau217. Effect sizes (rpb): eGFR <60 group = 0.48, CVD = 0.25, amyloid-PET-positive status = 0.54; tau-PET-positive status = 0.56. Lower eGFR was related to higher p-tau217 when adjusting for amyloid-PET. For abnormal amyloid-PET and tau-PET, p-tau217 exhibited areas under the curve of 0.90 and 0.89, respectively.
Discussion: Plasma p-tau217 showed promise as an Alzheimer's biomarker in Black adults; however, kidney function and CVD should be considered when interpreting levels.
Highlights: Plasma tau phosphorylated at threonine 217 (p-tau217) was tested in a sample of Black middle-aged and older adults. Level of p-tau217 was higher in impaired kidney function and cardiovascular disease. Obesity and diabetes were not related to p-tau217. Level of p-tau217 was higher in amyloid- and tau-PET-positive status. Plasma p-tau217 showed good receiver-operating characteristic area under the curve for abnormal amyloid- and tau-PET.
Keywords: African American; Alzheimer's disease; amyloid PET positivity; cardiovascular disease; estimated glomerular filtration rate; kidney function; medical comorbidities; plasma p‐tau217; tau‐PET positivity.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Carey E. Gleason is a scientific advisor for Huntington Research Institute, is a member of Alzheimer's Disease Research Center External Advisory Boards (Stanford University; University of New Mexico), is the Chair of the Institutional Data and Safety Monitoring Board for a National Institute on Aging (NIA)–funded study (R01‐AG074971), and has been a scientific advisor for a documentary film
Figures


References
MeSH terms
Substances
Grants and funding
- R01 AG074231/AG/NIA NIH HHS/United States
- R01 AG070940/AG/NIA NIH HHS/United States
- R01 AG062307/AG/NIA NIH HHS/United States
- R01-AG074231/National Institute on Aging - National Institutes of Health
- R01 AG080766/AG/NIA NIH HHS/United States
- 2023-00356/Swedish Research Council
- R01-AG062307/National Institute on Aging - National Institutes of Health
- ADSF-24-1284328-C/AD Strategic Fund
- 2022-01018/Swedish Research Council
- R56 AG062307/AG/NIA NIH HHS/United States
- UKDRI-1003/UK Dementia Research Institute
- FO2022-0270/Hjärnfonden
- 201809-2016862/Alzheimer's Drug Discovery Foundation
- ADSF-21-831381-C/AD Strategic Fund
- R03-AG063303/National Institute on Aging - National Institutes of Health
- 2019-02397/Swedish Research Council
- ALFGBG-71320/Swedish Research Council
- P30-AG062715/National Institute on Aging - National Institutes of Health
- Olav Thon Foundation
- ADSF-21-831377-C/AD Strategic Fund
- R01 AG027161/AG/NIA NIH HHS/United States
- R01-AG054059/National Institute on Aging - National Institutes of Health
- Erling-Persson Family Foundation
- R01 AG021155/AG/NIA NIH HHS/United States
- Bluefield Project
- R24-AG077433/National Institute on Aging - National Institutes of Health
- ADSF-21-831376-C/AD Strategic Fund
- R01 AG060737/AG/NIA NIH HHS/United States
- AARF-19-643973/ALZ/Alzheimer's Association/United States
- RF1 AG027161/AG/NIA NIH HHS/United States
- R24 AG077433/AG/NIA NIH HHS/United States
- R01-AG021155/National Institute on Aging - National Institutes of Health
- R01-AG027161/National Institute on Aging - National Institutes of Health
- JPND2021-00694/EU Joint Programme - Neurodegenerative Disease Research
- 101053962/HORIZON EUROPE Innovative Europe
- Stiftelsen för Gamla Tjänarinnor
- Cure Alzheimer's Fund
- P30 AG062715/AG/NIA NIH HHS/United States
- R01-AG060737/National Institute on Aging - National Institutes of Health
- R01-AG080766/National Institute on Aging - National Institutes of Health
- R01-AG070940/National Institute on Aging - National Institutes of Health
- R03 AG063303/AG/NIA NIH HHS/United States
- 860197/H2020 Marie Skłodowska-Curie Actions
- R01 AG054059/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

