Ubiquitin-proteasome system in the different stages of dominantly inherited Alzheimer's disease
- PMID: 40411302
- PMCID: PMC12102666
- DOI: 10.1002/alz.70243
Ubiquitin-proteasome system in the different stages of dominantly inherited Alzheimer's disease
Abstract
Introduction: This study investigated the role of the ubiquitin-proteasome system (UPS) in dominantly inherited Alzheimer's disease (DIAD) by examining cerebrospinal fluid (CSF) levels of UPS proteins.
Method: The SOMAscan assay was used to detect changes in UPS proteins in mutation carriers (MCs) relative to disease progression; imaging and CSF biomarkers of amyloid, tau, and neurodegeneration measures; and Clinical Dementia Rating scale.
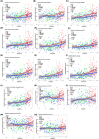
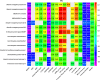
Results: Subtle increases in specific ubiquitin enzymes were detected in MCs up to two decades before symptom onset, with more pronounced elevations in UPS-activating enzymes near symptom onset. Significant correlations were found between UPS proteins and Alzheimer's disease (AD) biomarkers, especially between autophagy markers and late-stage tau biomarkers, microglia, and axonal degeneration.
Discussion: The rise in UPS proteins alongside tau-related markers suggests UPS involvement in tau neurofibrillary tangles. Elevated CSF UPS proteins in DIAD MCs may serve as indicators of disease progression, and may support the UPS as a therapeutic target in AD.
Highlights: This study investigates the ubiquitin-proteasome system (UPS) in Dominantly Inherited Alzheimer's Disease (DIAD), highlighting early molecular changes linked to disease progression. Using SOMAscan proteomics, we identified significant UPS protein alterations in cerebrospinal fluid of mutation carriers, notably up to 20 years before clinical symptom onset. Correlations between UPS protein levels and Alzheimer's biomarkers, particularly tau and neurodegeneration markers, suggest a strong association between UPS dysregulation and tau pathology in DIAD. Dynamic UPS changes align with A/T biological staging: UPS proteins were shown to increase across Aβ/tau (A/T) groups, with largest increases in the A+/T+ group, reinforcing their role in late-stage tau pathology and disease progression. These findings underscore the potential of UPS proteins as early biomarkers for Alzheimer's disease progression and as novel therapeutic targets, especially in tau-pathology-driven neurodegeneration. This work contributes to understanding AD pathogenesis, by emphasizing the importance of protein quality control systems and by offering avenues for future biomarker discovery and therapeutic development in Alzheimer's disease.
Keywords: amyloid beta; amyloid precursor protein; autophagy–lysosome pathway; biomarker discovery; dominantly inherited Alzheimer's disease; genetic mutations; neurodegeneration; presenilin 1; presenilin 2; protein aggregation; protein degradation; proteomic analysis; proteostasis; tau pathology; ubiquitin–proteasome system.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
RJB is the director of the DIAN‐TU and principal investigator of DIAN and the DIAN‐TU‐001 trial. Unrelated to this study, for the DIAN‐TU, he receives research support from the NIH, Eli Lilly and Company, F. Hoffman‐La Roche, Ltd., Eisai, Alzheimer's Association, GHR Foundation, Anonymous Organization, DIAN‐TU Pharma Consortium (Active Members: Biogen, Eisai, Eli Lilly and Company, Janssen, F. Hoffmann‐La Roche, Ltd./Genentech). JH is a paid consultant for F. Hoffmann‐La Roche, Ltd., Prothena, and Parabon Nanolabs, and is on a data safety and monitoring board (DSMB) for Eisai. EMM receives grant funding from NIA; Institutional funding from Eli Lilly, Hoffmann‐La Roche, Eisai. He is a DSMB member (paid directly) for Alector; Eli Lilly; a scientific advisory board member (paid directly to him) for Alzamend, Fondation Alzheimer. He acts as a consultant/advisor for Sage Therapeutics, Eli Lilly, Sanofi, AstraZeneca, Hoffmann La‐Roche. CC has received research support from GSK and EISAI. The funders of the study had no role in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. CC is a member of the advisory board of Circular Genomics and owns stocks in these companies. DP is an employee of GlaxoSmithKline (GSK) and holds stock in GSK. CX is supported by National Institute on Aging (NIA) grants R01 AG067505 and R01 AG053550. JCM is the Friedman Distinguished Professor of Neurology, Associate Director, Knight ADRC; Associate Director of DIAN, and Founding Principal Investigator of DIAN. He is funded by NIH grants # P30 AG066444; P01AG003991, P01AG026276, and U19 AG024904. Neither he nor his family owns stock or has equity interest (outside of mutual funds or other externally directed accounts) in any pharmaceutical or biotechnology company. TLSB has investigator‐initiated research funding from the NIH, the Alzheimer's Association, the Barnes‐Jewish Hospital Foundation, and Avid Radiopharmaceuticals. Dr. Benzinger participates as a site investigator in clinical trials sponsored by Avid Radiopharmaceuticals, Eli Lilly and Company, Biogen, Eisai, Jaansen, and F. Hoffmann‐La Roche, Ltd. She also serves as an unpaid consultant to Eisai and Siemens and is on the speaker's bureau for Biogen. AER reports no competing interests. He receives research support for this work from the National Institute on Aging (R01AG053267, U19AG032438). TI reports no competing interests. He received research support for this work from AMED (JP23dk0207066 and JP23dk0207049). GSD reports no competing interests directly relevant to this work. His research is supported by NIH (K23AG064029, U01AG057195, U01NS120901, U19AG032438). He serves as a consultant for Parabon Nanolabs Inc and as a topic editor (Dementia) for DynaMed (EBSCO). He is the co‐project PI for a clinical trial in anti‐NMDAR encephalitis, which receives support from Amgen Pharmaceuticals, and a consultant for Arialys Therapeutics. He has developed educational materials for PeerView Media, Inc., and Continuing Education Inc. He owns stock in ANI pharmaceuticals. Dr. Day's institution has received support from Eli Lilly for development and participation in an educational event promoting early diagnosis of symptomatic Alzheimer's disease, and in‐kind contributions of radiotracer precursors for tau‐PET neuroimaging in studies of memory and aging (via Avid Radiopharmaceuticals, a wholly owned subsidiary of Eli Lilly). RJP is Neuropathology Core Leader for the DIAN observational study and the DIAN Trials Unit. He receives research support for this work from the National Institute on Aging (U19 AG032438, U19AG032438‐09S1, R01AG068319). His laboratory receives cost recovery funding from Biogen for tissue procurement and processing services related to ALS clinical trials. Neither he nor his family owns stock or has equity interest (outside of mutual funds or other externally directed accounts) in any pharmaceutical or biotechnology company. FL has grants not related to this paper from NIH, DIAN, Enroll‐HD and BIOGEN. JL reports speaker fees from Bayer Vital, Biogen, EISAI, TEVA, Zambon, Esteve, Merck, and Roche; consulting fees from Axon Neuroscience, EISAI, and Biogen; author fees from Thieme medical publishers and W. Kohlhammer GmbH medical publishers; and is inventor in a patent “Oral Phenylbutyrate for Treatment of Human 4‐Repeat Tauopathies” (EP 23 156 122.6) filed by LMU Munich. In addition, he reports compensation for serving as chief medical officer for MODAG GmbH, is beneficiary of the phantom share program of MODAG GmbH, and is inventor in a patent “Pharmaceutical Composition and Methods of Use” (EP 22 159 408.8) filed by MODAG GmbH, all activities outside the submitted work. SBB receives support from the National Institute on Aging (NIA) and the Michael J. Fox Foundation. All other authors have nothing to disclose.
Figures


Update of
-
Ubiquitin-Proteasome System in the Different Stages of Dominantly Inherited Alzheimer's Disease.Res Sq [Preprint]. 2024 Jul 23:rs.3.rs-4202125. doi: 10.21203/rs.3.rs-4202125/v1. Res Sq. 2024. Update in: Alzheimers Dement. 2025 May;21(5):e70243. doi: 10.1002/alz.70243. PMID: 39108475 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
- P30 AG066444/AG/NIA NIH HHS/United States
- The Hope Center for Neurological Disorders
- R01AG078964/NH/NIH HHS/United States
- ZEN-22-848604/The Alzheimer's Association through the Zenith Fellows Award
- R01 AG074007/AG/NIA NIH HHS/United States
- Chan Zuckerberg Initiative (CZI)
- UL1TR002345/Washington University Institute of Clinical and Translational Sciences grant from National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health
- UL1 TR002345/TR/NCATS NIH HHS/United States
- R01AG064614/NH/NIH HHS/United States
- R01 AG078964/AG/NIA NIH HHS/United States
- P01 AG003991/AG/NIA NIH HHS/United States
- P01 AG026276/AG/NIA NIH HHS/United States
- RF1 AG058501/AG/NIA NIH HHS/United States
- R01 AG064614/AG/NIA NIH HHS/United States
- RF1AG058501/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

