Cis-nonProline peptides: Genuine occurrences and their functional roles
- PMID: 40411433
- PMCID: PMC12102755
- DOI: 10.1002/pro.70157
Cis-nonProline peptides: Genuine occurrences and their functional roles
Abstract
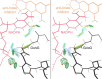
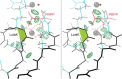
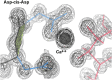
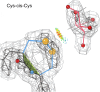
While cis peptides preceding proline can occur about 5% of the time, cis peptides preceding any other residue ("cis-nonPro" peptides) are an extremely rare feature in protein structures, of considerable importance for two opposite reasons. On one hand, their genuine occurrences are mostly found at sites critical to biological function, from the active sites of carbohydrate enzymes to rare adjacent-residue disulfide bonds. On the other hand, a cis-nonPro can easily be misfit into weak or ambiguous electron density, which led to a high incidence of unjustified cis-nonPro over the 2006-2015 decade. This paper uses high-resolution crystallographic data and especially stringent quality-filtering at the residue level to identify genuine occurrences of cis-nonPro and to survey both individual examples and broad patterns of their functionality. We explain the procedure developed to identify genuine cis-nonPro examples with almost no false positives. We then survey a large sample of the varied functional roles and structural contexts of cis-nonPro, including the uses of specific amino acids for particular purposes. We emphasize aspects not previously covered: that cis-nonPro essentially always (except for vicinal disulfides) occurs in well-ordered structure, and especially the great concentration of occurrence in proteins that process or bind carbohydrates (identified by occurrence on the CAZy website).
Keywords: Cys‐cis‐Cys; Gly‐cis‐Gly; Tyr‐cis‐X at active site in chitinases; asp‐cis‐asp; cis‐nonPro overrepresented in carbohydrate‐active enzymes; incorrect cis‐nonPro.
© 2025 The Author(s). Protein Science published by Wiley Periodicals LLC on behalf of The Protein Society.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Adrangi S, Faramarzi MA. From bacteria to human: a journey into the world of chitinases. Biotechnol Adv. 2013;13(8):1786–1795. - PubMed
-
- Bolin JT, Filman DJ, Matthews DA, Hamlin RC, Kraut J. Crystal structures of Escherichia coli and Lactobacillus casei dihydrofolate reductase refined at 1.7 Å resolution: I. General features and binding of methotrexate. J Biol Chem. 1982;257:13650–13662. - PubMed
-
- Brandts JF, Halvorsen HR, Brennan M. Consideration of the possibility that the slow step in protein denaturation reactions is due to cis‐trans isomerism of proline residues. Biochemistry. 1975;14:4953–4963. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

