Intraoperative Autologous Platelet-Rich Stroma Injection as Add-On to Fistula Curettage and Closure of the Internal Orifice Demonstrates a Favorable Outcome in Long-Term in Patients Suffering from Therapy-Refractory Perianal Fistulizing Crohn's Disease
- PMID: 40411444
- PMCID: PMC12455595
- DOI: 10.1093/ibd/izaf011
Intraoperative Autologous Platelet-Rich Stroma Injection as Add-On to Fistula Curettage and Closure of the Internal Orifice Demonstrates a Favorable Outcome in Long-Term in Patients Suffering from Therapy-Refractory Perianal Fistulizing Crohn's Disease
Abstract
Background: An injection with autologous platelet-rich stroma (PRS), a combination of stromal vascular fraction and platelet-rich plasma, as an add-on to fistula curretage and closure of the internal orifice proved to be safe and feasible for the treatment of patients with treatment-refractory perianal fistulizing Crohn's disease (pCD). This study aimed to assess the long-term outcomes in patients with pCD treated with autologous PRS injection.
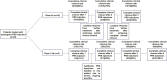
Methods: Adult patients with therapy-refractory pCD (failure to anti-tumor necrosis factor [TNF] therapy and/or fistula surgery), who underwent fistula curettage, closure of the internal fistula orifice, and autologous PRS injection in a Dutch tertiary referral center were included in an earlier conducted pilot study (n = 25). The primary outcome was complete clinical closure at long-term follow-up (closure of all treated external opening[s]). Secondary outcomes were partial clinical closure (closure of ≥1 treated external opening[s]), radiologic healing (fibrotic fistula tract on magnetic resonance imaging), and recurrence.
Results: The majority of the patients were female (56%) (mean age 34.4 years [standard deviation - SD: 0.9], and mean follow-up 3.7 years [SD: 0.6]). The treatment-refractory character of the study cohort was displayed by the high rate of patients with ≥1 external opening (60%), prior exposure to an anti-TNF agent (92%), TOpClass classification system ≥ class 2b (36%), and the low rate of patients who underwent prior surgical interventions aimed at fistula closure (12%). During long-term follow-up, complete clinical closure was achieved in 88%. Partial clinical closure was achieved in all patients. Radiologic healing was achieved in 75% of the patients. Recurrence was reported in 8% of the patients who achieved prior clinical closure. No recurrences were observed in patients with radiologic healing. Seventeen unplanned re-interventions were reported in nine patients (36%), predominantly for residual fistulizing disease and in patients with severe therapy-refractory pCD (TOpClass classification system ≥ class 2b) at the time of inclusion.
Conclusion: Additional PRS injection, fistula curettage, and closure of the internal orifice is a promising therapy for patients with (treatment-refractory) pCD and could improve clinical and radiologic healing rates. In addition, low recurrence rates were observed. Future randomized research is warranted in order to assess the effectiveness and positioning of PRS in the field of pCD.
Clinical trial registration: NL8417.
Keywords: Crohn’s disease; autologous; cell therapy; perianal fistula; platelet-rich plasma; platelet-rich stroma; stromal vascular fraction.
Plain language summary
This prospective pilot study is the first to assess the long-term outcomes of an additional injection of platelet-rich stroma, a combined product of stromal vascular fraction and platelet-rich plasma, to fistula curettage and closure of the internal orifice for patients with therapy-refractory perianal fistulizing Crohn’s disease.
© 2025 Crohn’s & Colitis Foundation. Published by Oxford University Press on behalf of Crohn’s & Colitis Foundation.
Conflict of interest statement
M.T.J. Bak has received speaker fees from AbbVie and an unrestricted research grant from Falk Pharma Benelux, outside the submitted work. A. C. de Vries has served on the advisory boards for Takeda, Janssen, Bristol Myers Squibb, Abbvie, Pfizer, and Galapagos; and has received unrestricted research grants from Takeda, Janssen, and Pfizer, outside the submitted work. C. J. van der Woude received grants and or fees for advisory boards and presentations from Pfizer, Abbvie, Celltrion, Falk Benelux, Takeda, Janssen, and Ferring, outside the submitted work. O. van Ruler has served as an invited speaker for Janssen-Cilag; and has received a research grant from Janssen and Takeda, outside the submitted work.
Figures


References
-
- Tsai L, McCurdy JD, Ma C, Jairath V, Singh S. Epidemiology and natural history of Perianal Crohn’s disease: a systematic review and meta-analysis of population-based cohorts. Inflamm Bowel Dis. 2022;28(10):1477-1484. doi: https://doi.org/ 10.1093/ibd/izab287 - DOI - PMC - PubMed
-
- Aguilera-Castro L, Ferre-Aracil C, Garcia-Garcia-de-Paredes A, Rodriguez-de-Santiago E, Lopez-Sanroman A. Management of complex perianal Crohn’s disease. Ann Gastroenterol. 2017;30(1):33-44. doi: https://doi.org/ 10.20524/aog.2016.0099 - DOI - PMC - PubMed
-
- Molendijk I, Nuij VJ, van der Meulen-de Jong AE, van der Woude CJ. Disappointing durable remission rates in complex Crohn’s disease fistula. Inflamm Bowel Dis. 2014;20(11):2022-2028. doi: https://doi.org/ 10.1097/MIB.0000000000000148 - DOI - PubMed
-
- Schwartz DA, Loftus EV, Jr, Tremaine WJ, et al. The natural history of fistulizing Crohn’s disease in Olmsted County, Minnesota. Gastroenterology. 2002;122(4):875-880. doi: https://doi.org/ 10.1053/gast.2002.32362 - DOI - PubMed
-
- Adamina M, Minozzi S, Warusavitarne J, et al. ECCO Guidelines on therapeutics in Crohn’s disease: surgical treatment. J Crohns Colitis. 2024;18(10):1556-1582. doi: https://doi.org/ 10.1093/ecco-jcc/jjae089 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

