Pharmacokinetic/Pharmacodynamic Modelling and Monte Carlo Simulations to Predict Cytomegalovirus Viral Load in Pediatric Transplant Recipients Treated with (val)Ganciclovir
- PMID: 40411698
- PMCID: PMC12185614
- DOI: 10.1007/s40262-025-01526-z
Pharmacokinetic/Pharmacodynamic Modelling and Monte Carlo Simulations to Predict Cytomegalovirus Viral Load in Pediatric Transplant Recipients Treated with (val)Ganciclovir
Abstract
Background and objectives: Cytomegalovirus (CMV) infection poses significant challenges in pediatric transplant recipients. Ganciclovir and its prodrug valganciclovir are primary treatments because of their potent antiviral effects. Balancing efficacy and toxicity is particularly critical in children. This study aimed to develop a pharmacokinetic/pharmacodynamic (PK/PD) model for (val)ganciclovir and assess the relationship between area under the concentration-time curve (AUC) and CMV viral loads via Monte Carlo simulations.
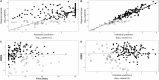
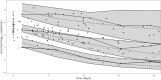
Methods: We conducted a retrospective analysis including 184 viral load samples from 36 transplanted children treated with ganciclovir/valganciclovir. We developed a population pharmacodynamic model using Monolix and performed Monte Carlo simulations to assess viral load decline with varying AUCs. Internal validation was performed using goodness-of-fit plots and bootstraps.
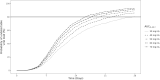
Results: We used a viral turnover model with stimulated degradation to model the pharmacodynamic data. Model validation showed no bias or misspecification. Simulations indicated that maintaining an AUC0-24 ≥ 40 mg·h/L achieved an 85.4% probability of undetectable viral load after 28 days of therapy. An AUC0-24 > 30 mg·h/L provided 80.9% probability of reducing viral loads by - 1 log after 2 weeks. AUC0-24 values > 60 mg·h/L offered minimal incremental benefits.
Conclusion: The pharmacodynamic model accurately predicted observed data. Simulations indicated that maintaining a ganciclovir plasma AUC0-24 around 40-60 mg·h/L maximized antiviral efficacy. An AUC0-24 > 60 mg·h/L might increase the risk of adverse events without providing additional efficacy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: Open access funding provided by Université de Limoges. Kévin Koloskoff received funding from the “association nationale recherche technologie” through a “convention industrielle de formation par la recherche” thesis (n°2021/1440). This research was also supported by ExactCure. Data availability: Supporting information is available in the ESM files, and supporting data and the Mlxtran model code are available from the authors on reasonable request. Conflicts of interest: The authors have no conflicts of interest. Ethics approval: The study protocol was approved by the institutional review board of CHU Sainte-Justine (reference number 2018-1830). Data were completely deidentified in accordance with the European General Data Protection Regulation. Consent to participate: Not applicable. Consent for publication: Not applicable. Authors contributions: KK wrote the manuscript. KK, JBW, and SB designed the research. All authors performed the research. KK, BF, JA, PO, and JBW analyzed the data.
Figures
References
-
- Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34(8):1094–7. - PubMed
-
- Kotton CN, Kumar D, Caliendo AM, Huprikar S, Chou S, Danziger-Isakov L, et al. The third international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2018;102:900–31. - PubMed
-
- Singh N. Preemptive therapy versus universal prophylaxis with ganciclovir for cytomegalovirus in solid organ transplant recipients. Clin Infect Dis. 2001;32:742–51. - PubMed
-
- Franck B, Autmizguine J, Marquet P, Ovetchkine P, Woillard J-B. Pharmacokinetics, pharmacodynamics, and therapeutic drug monitoring of valganciclovir and ganciclovir in transplantation. Clin Pharmacol Ther. 2022;112:233–76. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

