Escitalopram facilitates tumor growth and metastasis in rodents: Is it safe?
- PMID: 40411973
- PMCID: PMC12152346
- DOI: 10.1016/j.neo.2025.101182
Escitalopram facilitates tumor growth and metastasis in rodents: Is it safe?
Abstract
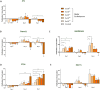
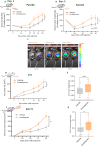
Cancer patients are often treated perioperatively with serotonin reuptake inhibitors (SSRIs) to counteract anxiety and depression. Recent studies suggest that long-term cancer outcomes may also be affected by SSRI use in an agent-dependent manner. Importantly, the perioperative use of SSRIs is prevalent clinically, but has rarely been studied empirically. Herein, we studied escitalopram, a commonly prescribed SSRI in cancer patients, in vitro, and in vivo in the context of surgery and/or cancer progression in immune-competent rodents, employing the Panc02 (pancreatic), MADB106, 4T1, EO771 (mammary), and CT26 (colon) syngeneic tumor models, assessing primary tumor growth and metastasis. Escitalopram (10-15mg/kg/day, 14-30 days) was administered along tumor and/or metastatic progression, via intraperitoneal injections, Alzet osmotic pumps, or drinking water. In vitro, escitalopram affected proliferation rates in a cell-line-, dose-, and exposure duration- dependent manner, mostly increasing or not affecting proliferation. In contrast, in vivo escitalopram consistently increased primary tumor growth, and experimental and spontaneous metastasis in all models tested. In pancreatic tumor-bearing mice, escitalopram increased tumor growth in two different studies by ∼1.5-fold, as indicated by bioluminescence imaging. In the mammary primary tumor models, escitalopram increased 4T1 and EO771 growth by 1.4 to 2.2-fold. Last, escitalopram increased experimental MADB106 lung metastasis and CT26 liver metastasis, as well as spontaneous post-excision 4T1 lung metastasis by 1.6 to 2.3-fold. Taken together, although additional research is needed to elucidate mediating in vivo mechanisms, and to assess clinical oncological risks of escitalopram, these findings raise concerns regarding the prevalent perioperative use of escitalopram in cancer patients.
Keywords: Cancer; Escitalopram; Metastasis; Periopearative period; Rodents.
Copyright © 2025. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Shamgar Ben-eliyahu reports financial support was provided by Israel Science Foundation. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

