Health-care burden related to respiratory syncytial virus in a resource-constrained setting: a prospective observational study
- PMID: 40412396
- PMCID: PMC12100462
- DOI: 10.1016/S2214-109X(25)00048-8
Health-care burden related to respiratory syncytial virus in a resource-constrained setting: a prospective observational study
Abstract
Background: Respiratory syncytial virus (RSV) is a leading cause of paediatric hospital admissions worldwide, straining health systems. A lack of data on the burden of RSV infections and the impact on health systems in resource-limited settings hinders evidence-based policy decisions. Here, we aimed to assess RSV's burden on the health system in Bangladesh.
Methods: From January to December, 2019, we conducted a prospective study at Bangladesh's largest paediatric hospital among children aged 0-59 months admitted with a possible respiratory infection, as guided by the WHO RSV hospital-based surveillance case definition. Outcomes for RSV-positive children younger than 5 years were analysed. We also followed up outcomes of children denied hospitalisation due to bed shortages. Adjusted hazard ratios for children denied admission versus admitted were estimated using survival analysis. Monte Carlo simulations with a queueing model were used to estimate the effects of RSV prefusion F maternal vaccine or nirsevimab on admission denials and mortality.
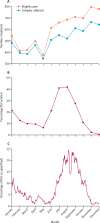
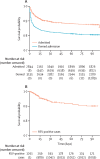
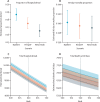
Findings: Of 40 664 children admitted, 31 692 were younger than 5 years; 19 940 were in study wards. Among 7191 admitted with possible respiratory infections, 6149 (85·5%) had nasopharyngeal swabs taken, with 1261 (20·5%) testing RSV-positive. The median age of children who tested positive for RSV was 3·0 months (IQR 1·0-8·0), with a median hospital stay of 5 days (IQR 4-8); 24 (1·9%) of 1261 died in hospital. 8274 (5·5%) of 151 110 bed days were for children who were positive for RSV. Additionally, of 9169 children denied admission, outcomes were tracked for 3928 and compared with 2845 admitted. The hazard ratio for death was 1·56 (95% CI 1·34 to 1·81) for children denied versus admitted, being highest for neonates at 2·27 (1·87 to 2·75). RSV prefusion F maternal vaccine or nirsevimab could have reduced denials by 677 (95% prediction interval 63 to 1347) and 1289 (684 to 1865), respectively, potentially preventing 130 (-60 to 322) and 258 (32 to 469) deaths.
Interpretation: RSV strains health care in Bangladesh, increasing mortality risks. Preventive interventions could lessen its impact, boosting health-care capacity and child health in resource-limited settings.
Funding: The Bill & Melinda Gates Foundation.
Copyright © 2025 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures
References
-
- Muller WJ, Madhi SA, Seoane Nuñez B, et al. Nirsevimab for prevention of RSV in term and late-preterm infants. N Engl J Med. 2023;388:1533–1534. - PubMed
-
- Hammitt LL, Dagan R, Yuan Y, et al. Nirsevimab for prevention of RSV in healthy late-preterm and term infants. N Engl J Med. 2022;386:837–846. - PubMed
-
- US Food and Drug Administration FDA approves first vaccine for pregnant individuals to prevent RSV in infants. Aug 21, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-v...
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

