IL-1β blockade prevents cardiotoxicity and improves the efficacy of immune checkpoint blockers and chemotherapy against pancreatic cancer in mice with obesity
- PMID: 40413022
- PMCID: PMC12104899
- DOI: 10.1136/jitc-2024-011404
IL-1β blockade prevents cardiotoxicity and improves the efficacy of immune checkpoint blockers and chemotherapy against pancreatic cancer in mice with obesity
Abstract
Background: Immune checkpoint blockers (ICBs) have revolutionized cancer therapy, yet they remain largely ineffective in treating pancreatic ductal adenocarcinoma (PDAC). Moreover, ICBs can cause severe immune-related adverse events (irAEs), including fatal cardiac toxicity. Finally, obesity is a risk factor in PDAC that may differentially modulate ICB efficacy in a malignancy-dependent manner.
Methods: We investigated the mechanisms underlying irAEs induced by dual ICB therapy and sought to identify strategies to mitigate them while improving ICB efficacy in the obese setting. To this end, we used a clinically relevant mouse model that integrated key features of human PDAC: (1) high-fat diet-induced obesity, (2) an orthotopic PDAC, and (3) a therapeutic regimen combining chemotherapy (FOLFIRINOX) with ICBs (α-programmed cell death protein-1 + α-cytotoxic T-lymphocyte associated protein-4 antibodies).
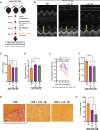
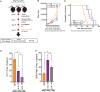
Results: Obese mice developed cardiac irAEs and had elevated serum interleukin (IL)-1β levels after chemoimmunotherapy. IL-1β blockade not only prevented myocarditis and reduced cardiac fibrosis but also enhanced the antitumor efficacy of the combination of chemotherapy plus dual ICB therapy and significantly improved the overall survival of PDAC-bearing obese mice.
Conclusions: Our findings provide the rationale and compelling data to test a Food and Drug Administration-approved anti-IL-1β antibody in combination with chemotherapy and dual ICB therapy in patients with pancreatic cancer with obesity.
Keywords: Cardiotoxicity; Immune Checkpoint Inhibitor; Immune related adverse event - irAE.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: RKJ received consultant fees from DynamiCure, SynDevRx; owns equity in Accurius, Enlight, SynDevRx; and served on the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund, Tekla World Healthcare Fund; and received a grant from Sanofi. DGD received research grants from Exelixis, BMS, Bayer, and Surface Oncology. MJP has served as a consultant for Acthera, ImmuneOncia, LegoChemBio, MaxiVax, Molecular Partners, Merck, Third Rock Ventures, UNIKUM, and Tidal. No funding or reagents from these companies were used in this study. The other authors have no competing interests to declare.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
