Avelumab-based neoadjuvant therapy in patients with muscle-invasive bladder cancer (AURA Oncodistinct-004): a phase 2 multicenter clinical trial
- PMID: 40413024
- PMCID: PMC12104948
- DOI: 10.1136/jitc-2025-012045
Avelumab-based neoadjuvant therapy in patients with muscle-invasive bladder cancer (AURA Oncodistinct-004): a phase 2 multicenter clinical trial
Abstract
Background: Immunotherapy is becoming a standard of care for non-metastatic muscle-invasive bladder cancer (MIBC). The optimal chemotherapy partner for chemo-immunotherapy combinations remains unknown. We evaluated the efficacy and safety of neoadjuvant avelumab-based regimens in patients with MIBC.
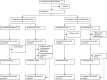
Methods: The multicenter phase 2 AURA trial (NCT03674424) enrolled patients with non-metastatic MIBC undergoing radical cystectomy. Cisplatin-eligible patients were randomized to receive avelumab with either dose-dense methotrexate-vinblastine-doxorubicin-cisplatin (ddMVAC-A) or gemcitabine-cisplatin (GC-A). Cisplatin-ineligible patients received either avelumab alone (A) or combined with paclitaxel-gemcitabine (PG-A). The primary endpoint was pathological complete response (pCR). Secondary endpoints included safety, event-free survival, and overall survival (OS).
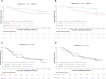
Results: Between July 2018 and September 2021, 137 eligible patients were enrolled in the trial. In the cisplatin-eligible cohort (n=79), pCR rates were 58% (95% CI: 42% to 72%) in the ddMVAC-A arm and 53% (95% CI: 37% to 68%) in the GC-A arm. The 36-month OS rates were 87% (95% CI: 76% to 98%) for ddMVAC-A and 67% (95% CI: 53% to 84%) for GC-A. In the cisplatin-ineligible cohort (n=58), pCR rates were 14% (95% CI: 6% to 31%) in the PG-A arm and 32% (95% CI: 18% to 51%) in the A arm. The 36-month OS rates were 48% (95% CI: 33% to 71%) for PG-A and 42% (95% CI: 27% to 65%) for A. Overall, 51 (38%) patients experienced grade 3-4 treatment-related adverse events.
Conclusions: Avelumab combined with cisplatin-based neoadjuvant chemotherapy showed promising efficacy in MIBC with a favorable safety profile, also with the ddMVAC regimen. Among cisplatin-ineligible patients, avelumab monotherapy showed encouraging activity, with no additional benefit observed from the PG-A regimen. These results support the use of the ddMVAC regimen as a potential chemotherapy partner for neoadjuvant chemo-immunotherapy combinations in future phase 3 trials, providing an alternative to the GC regimen currently under investigation.
Trial registration number: NCT03674424.
Keywords: Bladder Cancer; Genitourinary Cancer; Immune Checkpoint Inhibitor; Neoadjuvant.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: JB: FNRS funded PhD student, medical advisory (institutional) from Merck, travel support for medical congresses from Ipsen, Recordati, Merck and AstraZeneca. AC: honoraria for educational events from Astellas. LS: travel support by Merck. BS: honoraria from MSD and Janssen, consulting or advisory role for Astellas pharma, Janssen, BMS Belgium, Merck, Bayer, Johnson & Johnson/Janssen. NMC: research travel from Pfizer and Ipsen, consulting fees (institutional) from BMS, Bayer and Merck.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
