Alveolar epithelial and vascular CXCR2 mediates transcytosis of CXCL1 in inflamed lungs
- PMID: 40413164
- PMCID: PMC12103508
- DOI: 10.1038/s41467-025-60174-w
Alveolar epithelial and vascular CXCR2 mediates transcytosis of CXCL1 in inflamed lungs
Abstract
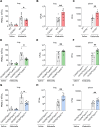
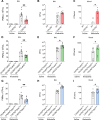
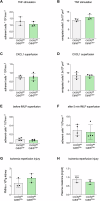
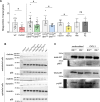
Pulmonary infections are characterized by neutrophil recruitment into the lung driven by chemokine ligands of CXCR2, which is expressed on neutrophils, but also present in non-hematopoietic lung cells, in which its role remains unclear. We hypothesize that CXCR2 in epithelial and endothelial cells contributes to neutrophil recruitment into the lung by modifying the availability of its cognate chemokines in lung alveoli. Using conditional endothelial and epithelial CXCR2 knockout mice, we demonstrate that selective CXCR2 deletion in either compartment impairs neutrophil recruitment into the lung during bacterial pneumonia and reduces bacterial clearance. We show that CXCR2 ablation in epithelial and endothelial cells compromises respective trans-epithelial and trans-endothelial transcytosis of alveolar CXCL1. Mechanistically, CXCR2-mediated CXCL1 endothelial and epithelial cell transcytosis requires the function of Bruton's tyrosine kinase in these cells. In conclusion, CXCR2 plays an important role in alveolar epithelial and endothelial cells, where it mediates cognate chemokine transcytosis, thus actively supporting their activities in neutrophil recruitment to the infected lungs.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





References
-
- Pober, J. S. & Sessa, W. C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol.7, 803–815 (2007). - PubMed
MeSH terms
Substances
Grants and funding
- CO2096/2-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- ZA428/18-2/Deutsche Forschungsgemeinschaft (German Research Foundation)
- INST211/1073-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- ZA428/14-2/Deutsche Forschungsgemeinschaft (German Research Foundation)
- INST 211/984-1/Deutsche Forschungsgemeinschaft (German Research Foundation)
- INST211/604-3/Deutsche Forschungsgemeinschaft (German Research Foundation)
- RO 4537/4-2/Deutsche Forschungsgemeinschaft (German Research Foundation)
- RO 4537/5-2/Deutsche Forschungsgemeinschaft (German Research Foundation)
- I-1470-412.13/2018/German-Israeli Foundation for Scientific Research and Development (GIF)
- I-1470-412.13/2018/German-Israeli Foundation for Scientific Research and Development (GIF)
- 200817/Z/16/Z/Wellcome Trust (Wellcome)
LinkOut - more resources
Full Text Sources

